Abstract
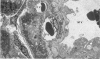
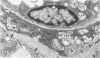
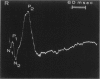
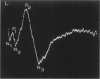
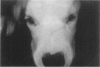
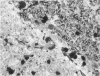
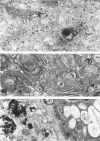
The clinical, morphologic, histochemical, and biochemical features of GM1-gangliosidosis in two canine models, English Springer Spaniel (ESS) and Portuguese Water Dog (PWD), have been compared. The disease onset, its clinical course, and survival period of the affected dogs were similar in both models. Skeletal dysplasia was noted radiographically at 2 months of age, whereas at 4 1/2 months of age there was progressive neurologic impairment. However, dwarfism and coarse facial features were seen only in ESS. Both models had similar deficiency in activity of lysosomal beta-galactosidase, but possessed a normal protein activator for GM1-beta-galactosidase. Both models stored GM1-ganglioside, asialo-GM1, and oligosaccharides in brain. Furthermore, only the PWD stored glycoproteins containing polylactosaminoglycans in visceral organs, and neither model stored them in the brain. Morphologically, both models demonstrated similar storage material in multiple tissues and cell types. The ultrastructure of the storage material was cell-type specific and identical in both models. However, some differences in the lectin staining pattern were noted. Our clinical, biochemical, and histochemical findings indicate that PWD and ESS may represent two different mutations of the beta-galactosidase gene. Moreover, the authors conclude that it is difficult, and inappropriate, to apply the human classification of GM1-gangliosidosis (i.e. infantile, juvenile, and adult forms) to these canine models.
Full text
PDF



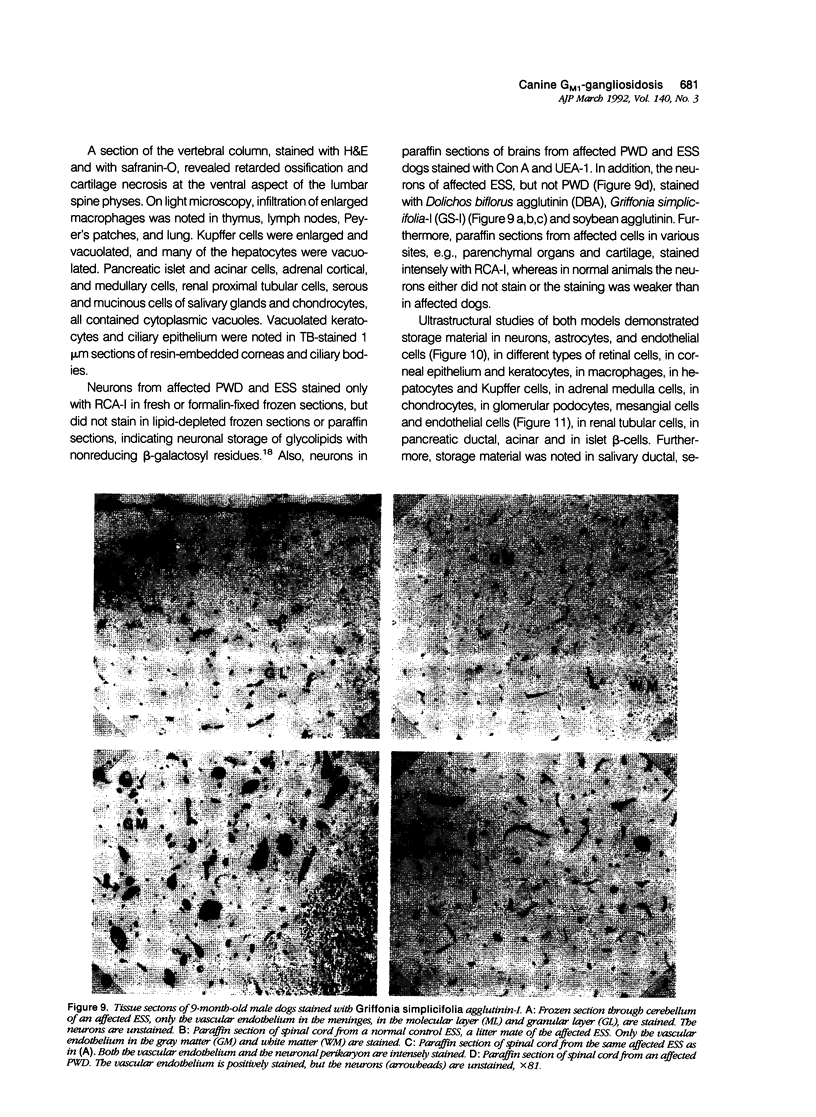
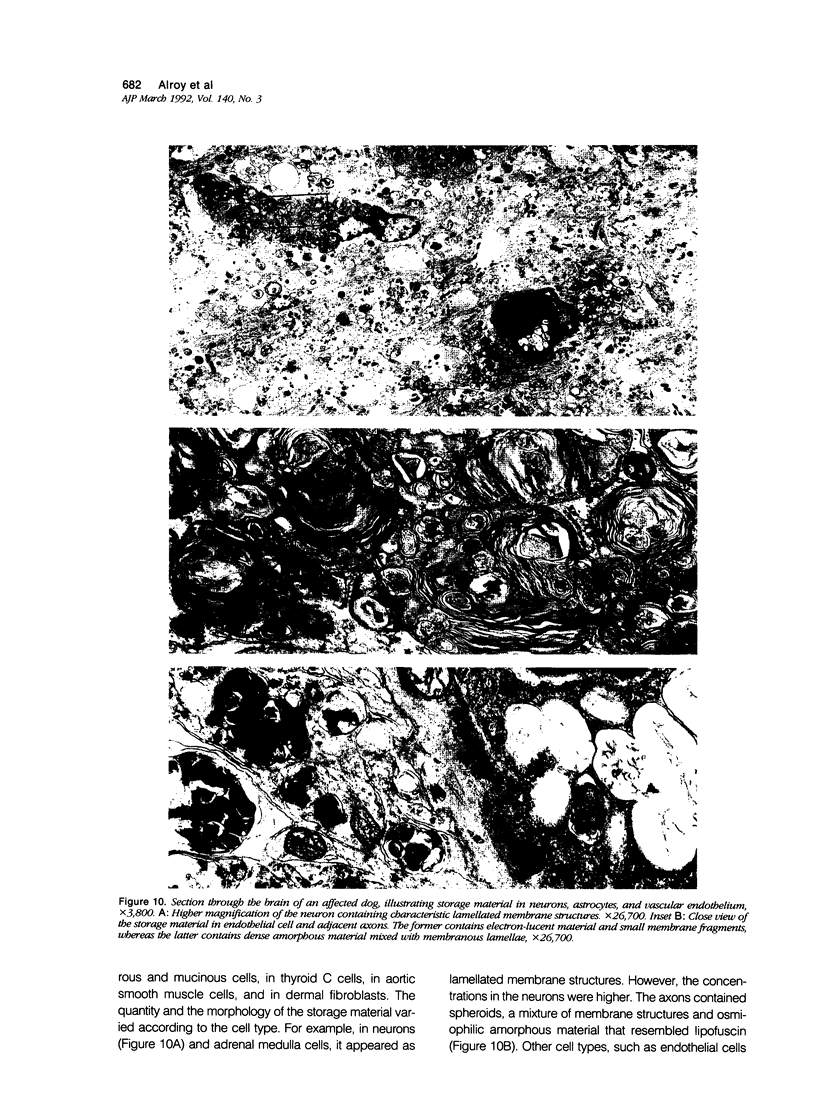
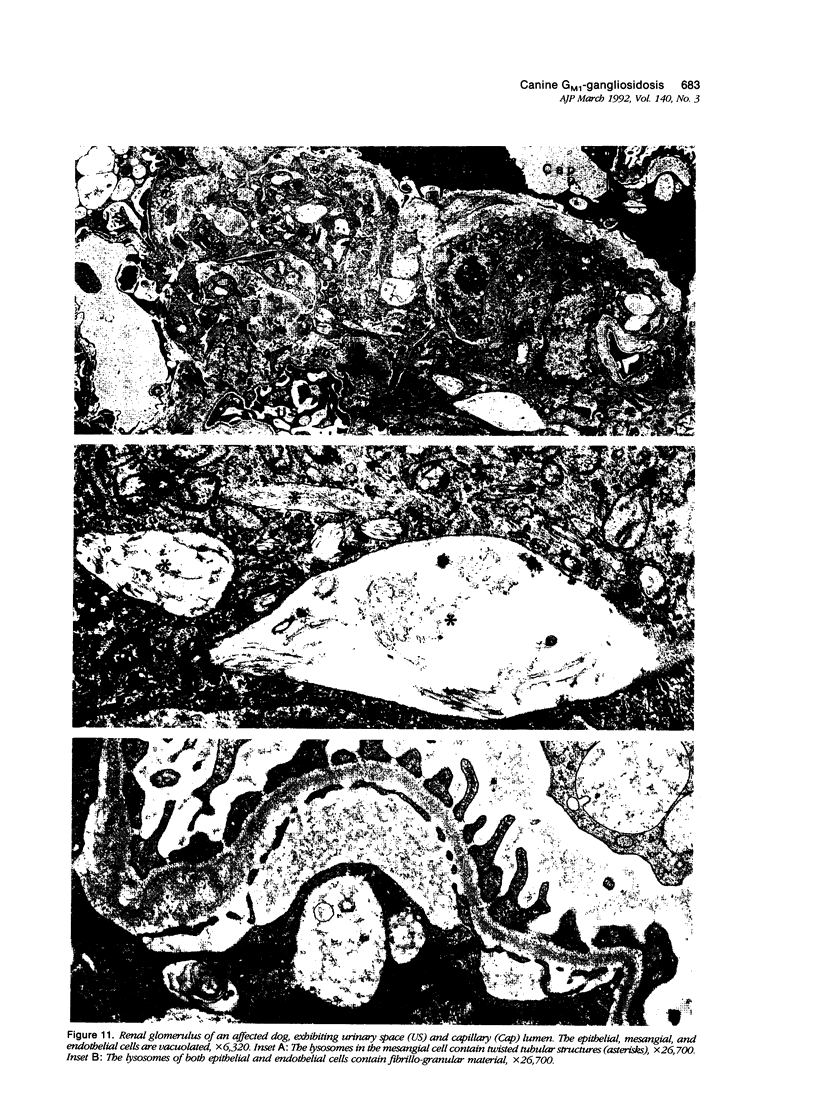

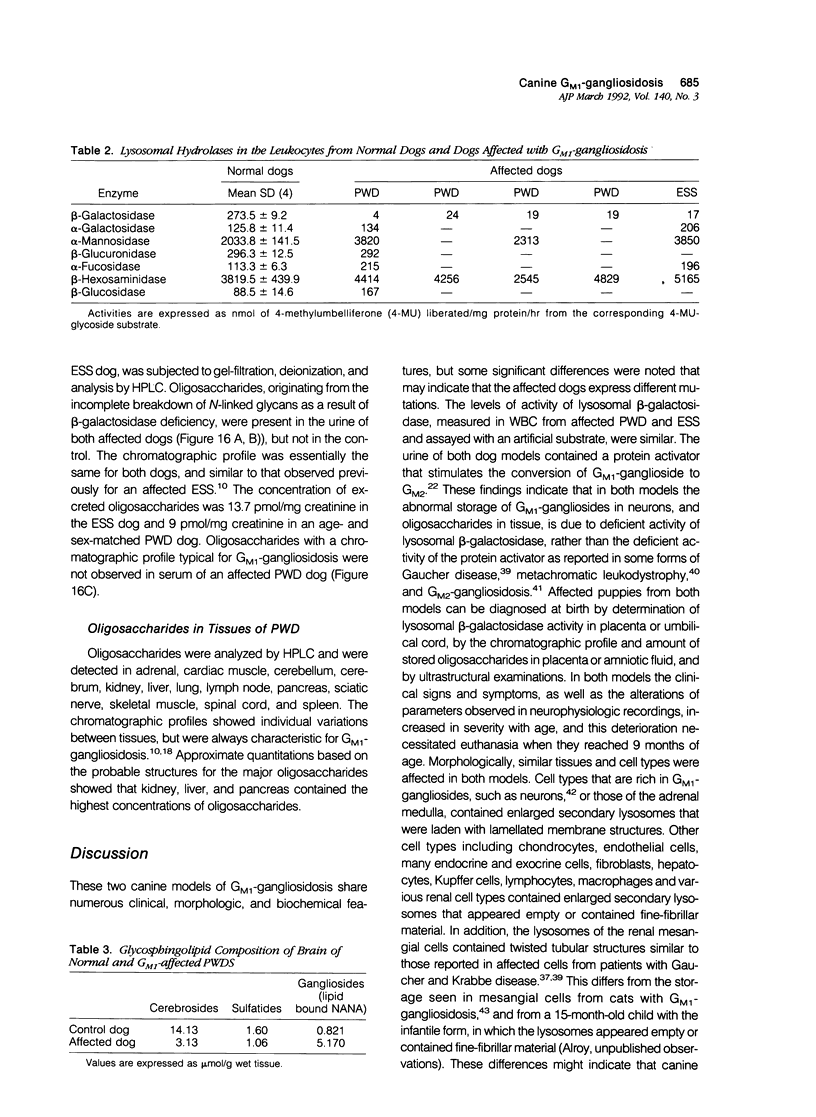


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMINOFF D. Methods for the quantitative estimation of N-acetylneuraminic acid and their application to hydrolysates of sialomucoids. Biochem J. 1961 Nov;81:384–392. doi: 10.1042/bj0810384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahern-Rindell A. J., Murnane R. D., Prieur D. J. Interspecific genetic complementation analysis of human and sheep fibroblasts with beta-galactosidase deficiency. Somat Cell Mol Genet. 1989 Nov;15(6):525–533. doi: 10.1007/BF01534913. [DOI] [PubMed] [Google Scholar]
- Ahern-Rindell A. J., Prieur D. J., Murnane R. D., Raghavan S. S., Daniel P. F., McCluer R. H., Walkley S. U., Parish S. M. Inherited lysosomal storage disease associated with deficiencies of beta-galactosidase and alpha-neuraminidase in sheep. Am J Med Genet. 1988 Sep;31(1):39–56. doi: 10.1002/ajmg.1320310108. [DOI] [PubMed] [Google Scholar]
- Alroy J., Bachrach A., Jr, Thalhammer J. G., Panjwani N., Richard R., DeGasperi R., Warren C. D., Albert D. M., Raghavan S. S. Clinical, neurophysiological, biochemical and morphological features of eyes in Persian cats with mannosidosis. Virchows Arch B Cell Pathol Incl Mol Pathol. 1991;60(3):173–180. doi: 10.1007/BF02899544. [DOI] [PubMed] [Google Scholar]
- Alroy J., De Gasperi R., Warren C. D. Application of lectin histochemistry and carbohydrate analysis to the characterization of lysosomal storage diseases. Carbohydr Res. 1991 Jun 25;213:229–250. doi: 10.1016/s0008-6215(00)90611-6. [DOI] [PubMed] [Google Scholar]
- Alroy J., Goyal V., Warren C. D. Lectin histochemistry of gangliosidosis. I. Neural tissue in four mammalian species. Acta Neuropathol. 1988;76(2):109–114. doi: 10.1007/BF00688094. [DOI] [PubMed] [Google Scholar]
- Alroy J., Orgad U., Ucci A. A., Schelling S. H., Schunk K. L., Warren C. D., Raghavan S. S., Kolodny E. H. Neurovisceral and skeletal GM1-gangliosidosis in dogs with beta-galactosidase deficiency. Science. 1985 Aug 2;229(4712):470–472. doi: 10.1126/science.3925555. [DOI] [PubMed] [Google Scholar]
- Alroy J., Ucci A. A., Goyal V., Woods W. Lectin histochemistry of glycolipid storage diseases on frozen and paraffin-embedded tissue sections. J Histochem Cytochem. 1986 Apr;34(4):501–505. doi: 10.1177/34.4.3081625. [DOI] [PubMed] [Google Scholar]
- Alroy J., Warren C. D., Raghavan S. S., Daniel P. F., Schunk K. L., Kolodny E. H. Biochemical, ultrastructural and histochemical studies of cat placentae deficient in activity of lysosomal alpha-mannosidase. Placenta. 1987 Sep-Oct;8(5):545–553. doi: 10.1016/0143-4004(87)90083-x. [DOI] [PubMed] [Google Scholar]
- Baker H. J., Jr, Lindsey J. R., McKhann G. M., Farrell D. F. Neuronal GM1 gangliosidosis in a Siamese cat with beta-galactosidase deficiency. Science. 1971 Nov 19;174(4011):838–839. doi: 10.1126/science.174.4011.838. [DOI] [PubMed] [Google Scholar]
- Berra B., De Gasperi R., Rapelli S., Okada S., Li S. C., Li Y. T. Presence of glycoproteins containing the polylactosamine structure in brain and liver of GM1 gangliosidosis patients. Comparative study between clinical types I and II, using endo-beta-galactosidase enzyme. Neurochem Pathol. 1986 Apr;4(2):107–117. doi: 10.1007/BF03160189. [DOI] [PubMed] [Google Scholar]
- Blakemore W. F. GM-1 gangliosidosis in a cat. J Comp Pathol. 1972 Apr;82(2):179–185. doi: 10.1016/0021-9975(72)90061-8. [DOI] [PubMed] [Google Scholar]
- Castagnaro M., Alroy J., Ucci A. A., Glew R. H. Lectin histochemistry and ultrastructure of feline kidneys from six different storage diseases. Virchows Arch B Cell Pathol Incl Mol Pathol. 1987;54(1):16–26. doi: 10.1007/BF02899193. [DOI] [PubMed] [Google Scholar]
- Dahl D. L., Warren C. D., Rathke E. J., Jones M. Z. Beta-mannosidosis: prenatal detection of caprine allantoic fluid oligosaccharides with thin layer, gel permeation and high performance liquid chromatography. J Inherit Metab Dis. 1986;9(1):93–98. doi: 10.1007/BF01813910. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Jolly R. D., Slack P. M., Winter P. J., Murphy C. E. Mannosidosis: patterns of storage and urinary excretion of oligosaccharides in the bovine model. Aust J Exp Biol Med Sci. 1980 Aug;58(4):421–428. doi: 10.1038/icb.1980.43. [DOI] [PubMed] [Google Scholar]
- Kasama T., Taketomi T. Abnormalities of cerebral lipids in GM1-gangliosidoses, infantile, juvenile, and chronic type. Jpn J Exp Med. 1986 Feb;56(1):1–11. [PubMed] [Google Scholar]
- Kleinschmidt T., Christomanou H., Braunitzer G. Complete amino-acid sequence and carbohydrate content of the naturally occurring glucosylceramide activator protein (A1 activator) absent from a new human Gaucher disease variant. Biol Chem Hoppe Seyler. 1987 Dec;368(12):1571–1578. doi: 10.1515/bchm3.1987.368.2.1571. [DOI] [PubMed] [Google Scholar]
- LANDING B. H., SILVERMAN F. N., CRAIG J. M., JACOBY M. D., LAHEY M. E., CHADWICK D. L. FAMILIAL NEUROVISCERAL LIPIDOSIS. AN ANALYSIS OF EIGHT CASES OF A SYNDROME PREVIOUSLY REPORTED AS "HURLER-VARIANT," "PSEUDO-HURLER," AND "TAY-SACHS DISEASE WITH VISCERAL INVOLVEMENT". Am J Dis Child. 1964 Nov;108:503–522. [PubMed] [Google Scholar]
- Ledeen R. W., Yu R. K. Gangliosides: structure, isolation, and analysis. Methods Enzymol. 1982;83:139–191. doi: 10.1016/0076-6879(82)83012-7. [DOI] [PubMed] [Google Scholar]
- Lee R. E. The fine structure of the cerebroside occurring in Gaucher's disease. Proc Natl Acad Sci U S A. 1968 Oct;61(2):484–489. doi: 10.1073/pnas.61.2.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S. C., Kihara H., Serizawa S., Li Y. T., Fluharty A. L., Mayes J. S., Shapiro L. J. Activator protein required for the enzymatic hydrolysis of cerebroside sulfate. Deficiency in urine of patients affected with cerebroside sulfatase activator deficiency and identity with activators for the enzymatic hydrolysis of GM1 ganglioside and globotriaosylceramide. J Biol Chem. 1985 Feb 10;260(3):1867–1871. [PubMed] [Google Scholar]
- Li Y. T., Muhiudeen I. A., DeGasperi R., Hirabayashi Y., Li S. C. Presence of activator proteins for the enzymic hydrolysis of GM1 and GM2 gangliosides in normal human urine. Am J Hum Genet. 1983 Jul;35(4):629–634. [PMC free article] [PubMed] [Google Scholar]
- Lott I. T., Daniel P. F. Serum and urinary trisaccharides in mannosidosis. Neurology. 1981 Sep;31(9):1159–1162. [PubMed] [Google Scholar]
- Murnane R. D., Ahern-Rindell A. J., Prieur D. J. Lectin histochemistry of an ovine lysosomal storage disease with deficiencies of beta-galactosidase and alpha-neuraminidase. Am J Pathol. 1989 Oct;135(4):623–630. [PMC free article] [PubMed] [Google Scholar]
- Murnane R. D., Prieur D. J., Ahern-Rindell A. J., Parish S. M., Collier L. L. The lesions of an ovine lysosomal storage disease. Initial characterization. Am J Pathol. 1989 Feb;134(2):263–270. [PMC free article] [PubMed] [Google Scholar]
- Neskovic N., Sarlieve L., Nussbaum J. L., Kostic D., Mandel P. Quantitative thin-layer chromatography of glycolipids in animal tissues. Clin Chim Acta. 1972 Apr;38(1):147–153. doi: 10.1016/0009-8981(72)90220-3. [DOI] [PubMed] [Google Scholar]
- Read D. H., Harrington D. D., Keenana T. W., Hinsman E. J. Neuronal-visceral GM1 gangliosidosis in a dog with beta-galactosidase deficiency. Science. 1976 Oct 22;194(4263):442–445. doi: 10.1126/science.824730. [DOI] [PubMed] [Google Scholar]
- Rodriguez M., O'Brien J. S., Garrett R. S., Powell H. C. Canine GM1 gangliosidosis. An ultrastructural and biochemical study. J Neuropathol Exp Neurol. 1982 Nov;41(6):618–629. doi: 10.1097/00005072-198211000-00005. [DOI] [PubMed] [Google Scholar]
- Roth J. Cytochemical localization of terminal N-acetyl-D-galactosamine residues in cellular compartments of intestinal goblet cells: implications for the topology of O-glycosylation. J Cell Biol. 1984 Feb;98(2):399–406. doi: 10.1083/jcb.98.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders G. K., Wood P. A., Myers R. K., Shell L. G., Carithers R. GM1 gangliosidosis in Portuguese water dogs: pathologic and biochemical findings. Vet Pathol. 1988 Jul;25(4):265–269. doi: 10.1177/030098588802500403. [DOI] [PubMed] [Google Scholar]
- Shell L. G., Potthoff A. I., Carithers R., Katherman A., Saunders G. K., Wood P. A., Giger U. Neuronal-visceral GM1 gangliosidosis in Portuguese water dogs. J Vet Intern Med. 1989 Jan-Mar;3(1):1–7. doi: 10.1111/j.1939-1676.1989.tb00320.x. [DOI] [PubMed] [Google Scholar]
- Warner T. G., O'Brien J. S. Structure analysis of the major oligosaccharides accumulating in canine GM1 gangliosidosis liver. J Biol Chem. 1982 Jan 10;257(1):224–232. [PubMed] [Google Scholar]
- Warner T. G., Robertson A. D., O'Brien J. S. Diagnosis of GM1 gangliosidosis based on detection of urinary oligosaccharides with high performance liquid chromatography. Clin Chim Acta. 1983 Feb 7;127(3):313–326. doi: 10.1016/0009-8981(83)90158-4. [DOI] [PubMed] [Google Scholar]
- Warren C. D., Alroy J., Bugge B., Daniel P. F., Raghavan S. S., Kolodny E. H., Lamar J. J., Jeanloz R. W. Oligosaccharides from placenta: early diagnosis of feline mannosidosis. FEBS Lett. 1986 Jan 20;195(1-2):247–252. doi: 10.1016/0014-5793(86)80169-7. [DOI] [PubMed] [Google Scholar]
- Williams M. A., Gross S. K., Evans J. E., McCluer R. H. Glycolipid stage-specific embryonic antigens (SSEA-1) in kidneys of male and female C57BL/6J and beige adult mice. J Lipid Res. 1988 Dec;29(12):1613–1619. [PubMed] [Google Scholar]
- Williams M. A., McCluer R. H. The use of Sep-Pak C18 cartridges during the isolation of gangliosides. J Neurochem. 1980 Jul;35(1):266–269. doi: 10.1111/j.1471-4159.1980.tb12515.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Boyer A. M., Schwarting G. A. Fucose-containing glycolipids are stage- and region-specific antigens in developing embryonic brain of rodents. Proc Natl Acad Sci U S A. 1985 May;82(9):3045–3049. doi: 10.1073/pnas.82.9.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunis E. J., Lee R. E. The ultrastructure of globoid (Krabbe) leukodystrophy. Lab Invest. 1969 Nov;21(5):415–419. [PubMed] [Google Scholar]