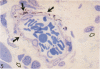
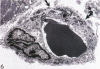
Abstract
Acute inflammation is characterized mainly by the egress of neutrophils from postcapillary venules and by increased vascular permeability leading to the formation of edema. The microvascular site of increased vascular permeability in local acute inflammatory lesions was investigated after the injection of endotoxin (LPS), interleukin-1 (IL-1), and tumor necrosis factor (TNF) into the dermis overlying the cremasteric and rectus abdominis muscles of rats. LPS caused leakage of colloidal carbon peaking at 3 to 4 hours at the level of the postcapillary venules and capillary leak was variably observed at later time points. IL-1 and TNF also caused postcapillary venular leakage. IL-1 was as potent as LPS and more so than TNF. The microvascular leak caused by LPS, IL-1, and TNF was accompanied by the tissue accumulation of neutrophils, and was neutrophil-dependent because LPS, IL-1, and TNF did not cause vascular labelling in neutropenic rats.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arfors K. E., Rutili G., Svensjö E. Microvascular transport of macromolecules in normal and inflammatory conditions. Acta Physiol Scand Suppl. 1979;463:93–103. [PubMed] [Google Scholar]
- Claudio L., Kress Y., Factor J., Brosnan C. F. Mechanisms of edema formation in experimental autoimmune encephalomyelitis. The contribution of inflammatory cells. Am J Pathol. 1990 Nov;137(5):1033–1045. [PMC free article] [PubMed] [Google Scholar]
- Cybulsky M. I., Chan M. K., Movat H. Z. Acute inflammation and microthrombosis induced by endotoxin, interleukin-1, and tumor necrosis factor and their implication in gram-negative infection. Lab Invest. 1988 Apr;58(4):365–378. [PubMed] [Google Scholar]
- Goldblum S. E., Hennig B., Jay M., Yoneda K., McClain C. J. Tumor necrosis factor alpha-induced pulmonary vascular endothelial injury. Infect Immun. 1989 Apr;57(4):1218–1226. doi: 10.1128/iai.57.4.1218-1226.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldie P., Hellström S. Identification and characterization of middle ear vascular leakage sites in experimental otitis media. Ann Otol Rhinol Laryngol. 1990 Oct;99(10 Pt 1):810–816. doi: 10.1177/000348949009901011. [DOI] [PubMed] [Google Scholar]
- Hocking D. C., Phillips P. G., Ferro T. J., Johnson A. Mechanisms of pulmonary edema induced by tumor necrosis factor-alpha. Circ Res. 1990 Jul;67(1):68–77. doi: 10.1161/01.res.67.1.68. [DOI] [PubMed] [Google Scholar]
- Horvath C. J., Ferro T. J., Jesmok G., Malik A. B. Recombinant tumor necrosis factor increases pulmonary vascular permeability independent of neutrophils. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9219–9223. doi: 10.1073/pnas.85.23.9219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joris I., Cuénoud H. F., Doern G. V., Underwood J. M., Majno G. Capillary leakage in inflammation. A study by vascular labeling. Am J Pathol. 1990 Dec;137(6):1353–1363. [PMC free article] [PubMed] [Google Scholar]
- Joris I., Majno G., Corey E. J., Lewis R. A. The mechanism of vascular leakage induced by leukotriene E4. Endothelial contraction. Am J Pathol. 1987 Jan;126(1):19–24. [PMC free article] [PubMed] [Google Scholar]
- Kopaniak M. M., Issekutz A. C., Movat H. Z. Kinetics of acute inflammation induced by E coli in rabbits. Quantitation of blood flow, enhanced vascular permeability, hemorrhage, and leukocyte accumulation. Am J Pathol. 1980 Feb;98(2):485–498. [PMC free article] [PubMed] [Google Scholar]
- Kopaniak M. M., Movat H. Z. Kinetics of acute inflammation induced by Escherichia coli in rabbits. II. The effect of hyperimmunization, complement depletion, and depletion of leukocytes. Am J Pathol. 1983 Jan;110(1):13–29. [PMC free article] [PubMed] [Google Scholar]
- MAJNO G., PALADE G. E., SCHOEFL G. I. Studies on inflammation. II. The site of action of histamine and serotonin along the vascular tree: a topographic study. J Biophys Biochem Cytol. 1961 Dec;11:607–626. doi: 10.1083/jcb.11.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAJNO G., PALADE G. E. Studies on inflammation. 1. The effect of histamine and serotonin on vascular permeability: an electron microscopic study. J Biophys Biochem Cytol. 1961 Dec;11:571–605. doi: 10.1083/jcb.11.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majno G., Shea S. M., Leventhal M. Endothelial contraction induced by histamine-type mediators: an electron microscopic study. J Cell Biol. 1969 Sep;42(3):647–672. doi: 10.1083/jcb.42.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movat H. Z., Burrowes C. E., Cybulsky M. I., Dinarello C. A. Acute inflammation and a Shwartzman-like reaction induced by interleukin-1 and tumor necrosis factor. Synergistic action of the cytokines in the induction of inflammation and microvascular injury. Am J Pathol. 1987 Dec;129(3):463–476. [PMC free article] [PubMed] [Google Scholar]
- Munro J. M., Pober J. S., Cotran R. S. Recruitment of neutrophils in the local endotoxin response: association with de novo endothelial expression of endothelial leukocyte adhesion molecule-1. Lab Invest. 1991 Feb;64(2):295–299. [PubMed] [Google Scholar]
- Pober J. S. Warner-Lambert/Parke-Davis award lecture. Cytokine-mediated activation of vascular endothelium. Physiology and pathology. Am J Pathol. 1988 Dec;133(3):426–433. [PMC free article] [PubMed] [Google Scholar]
- Pober J., Cotran R. S. What can be learned from the expression of endothelial adhesion molecules in tissues? Lab Invest. 1991 Mar;64(3):301–305. [PubMed] [Google Scholar]
- Rampart M., De Smet W., Fiers W., Herman A. G. Inflammatory properties of recombinant tumor necrosis factor in rabbit skin in vivo. J Exp Med. 1989 Jun 1;169(6):2227–2232. doi: 10.1084/jem.169.6.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum J. T., Howes E. L., Jr, Rubin R. M., Samples J. R. Ocular inflammatory effects of intravitreally-injected tumor necrosis factor. Am J Pathol. 1988 Oct;133(1):47–53. [PMC free article] [PubMed] [Google Scholar]
- Simionescu M., Simionescu N., Palade G. E. Segmental differentiations of cell junctions in the vascular endothelium. The microvasculature. J Cell Biol. 1975 Dec;67(3):863–885. doi: 10.1083/jcb.67.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simionescu N., Simionescu M., Palade G. E. Open junctions in the endothelium of the postcapillary venules of the diaphragm. J Cell Biol. 1978 Oct;79(1):27–44. doi: 10.1083/jcb.79.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens K. E., Ishizaka A., Wu Z. H., Larrick J. W., Raffin T. A. Granulocyte depletion prevents tumor necrosis factor-mediated acute lung injury in guinea pigs. Am Rev Respir Dis. 1988 Nov;138(5):1300–1307. doi: 10.1164/ajrccm/138.5.1300. [DOI] [PubMed] [Google Scholar]
- Ulich T. R., Watson L. R., Yin S. M., Guo K. Z., Wang P., Thang H., del Castillo J. The intratracheal administration of endotoxin and cytokines. I. Characterization of LPS-induced IL-1 and TNF mRNA expression and the LPS-, IL-1-, and TNF-induced inflammatory infiltrate. Am J Pathol. 1991 Jun;138(6):1485–1496. [PMC free article] [PubMed] [Google Scholar]