Abstract
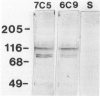
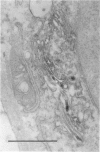
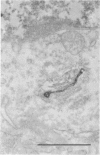
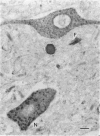
The Golgi apparatus (complex) is at the center stage of important functions of processing and transport of plasma membrane, lysosomal, and secreted proteins. The involvement of the Golgi apparatus in the pathogenesis of chronic degenerative diseases of neurons is virtually unknown. In the present study, fragmentation and atrophy of the Golgi apparatus of motor neurons in amyotrophic lateral sclerosis (ALS), has been detected with organelle specific antibodies. Approximately 30% of motor neurons in five ALS patients showed a fragmented Golgi apparatus whereas only about 1% of motor neurons from seven controls with neurologic or systemic disease showed a similar change. Morphometric studies are consistent with the hypothesis that the alteration of the Golgi apparatus is an early event in the pathogenesis of the neuronal degeneration in ALS. Immunocytochemical studies with antibodies against alpha tubulin, tau, and phosphorylated subunits of neurofilament polypeptides did not disclose differences in the staining of neurons with fragmented or normal Golgi apparatus, suggesting that the alteration of the organelle is not secondary to a gross lesion of the cytoskeleton. However, these observations do not rule out the hypothesis that the fragmentation of the Golgi apparatus is secondary to subtle changes of the polypeptides involved in the attachment of membranes of the organelle to the cytoskeleton.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appel S. H. A unifying hypothesis for the cause of amyotrophic lateral sclerosis, parkinsonism, and Alzheimer disease. Ann Neurol. 1981 Dec;10(6):499–505. doi: 10.1002/ana.410100602. [DOI] [PubMed] [Google Scholar]
- Appel S. H., Engelhardt J. I., García J., Stefani E. Immunoglobulins from animal models of motor neuron disease and from human amyotrophic lateral sclerosis patients passively transfer physiological abnormalities to the neuromuscular junction. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):647–651. doi: 10.1073/pnas.88.2.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barde Y. A. Trophic factors and neuronal survival. Neuron. 1989 Jun;2(6):1525–1534. doi: 10.1016/0896-6273(89)90040-8. [DOI] [PubMed] [Google Scholar]
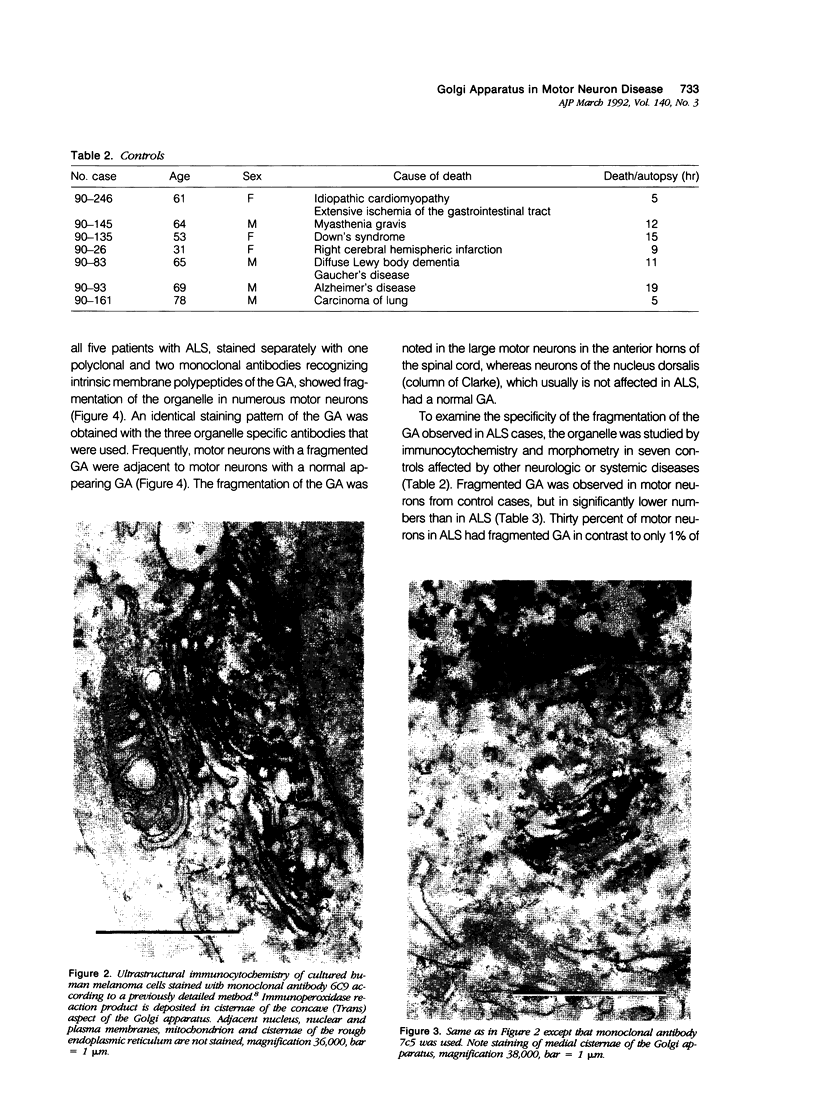
- Croul S. E., Mezitis S. G., Gonatas N. K. An anti-organelle antibody in pathology. The chromatolytic reaction studied with a monoclonal antibody against the Golgi apparatus. Am J Pathol. 1988 Nov;133(2):355–362. [PMC free article] [PubMed] [Google Scholar]
- Croul S., Mezitis S. G., Stieber A., Chen Y. J., Gonatas J. O., Goud B., Gonatas N. K. Immunocytochemical visualization of the Golgi apparatus in several species, including human, and tissues with an antiserum against MG-160, a sialoglycoprotein of rat Golgi apparatus. J Histochem Cytochem. 1990 Jul;38(7):957–963. doi: 10.1177/38.7.2355176. [DOI] [PubMed] [Google Scholar]
- Delbono O., García J., Appel S. H., Stefani E. IgG from amyotrophic lateral sclerosis affects tubular calcium channels of skeletal muscle. Am J Physiol. 1991 Jun;260(6 Pt 1):C1347–C1351. doi: 10.1152/ajpcell.1991.260.6.C1347. [DOI] [PubMed] [Google Scholar]
- Engelhardt J. I., Appel S. H. IgG reactivity in the spinal cord and motor cortex in amyotrophic lateral sclerosis. Arch Neurol. 1990 Nov;47(11):1210–1216. doi: 10.1001/archneur.1990.00530110068019. [DOI] [PubMed] [Google Scholar]
- Farquhar M. G. Progress in unraveling pathways of Golgi traffic. Annu Rev Cell Biol. 1985;1:447–488. doi: 10.1146/annurev.cb.01.110185.002311. [DOI] [PubMed] [Google Scholar]
- Gonatas J. O., Mezitis S. G., Stieber A., Fleischer B., Gonatas N. K. MG-160. A novel sialoglycoprotein of the medial cisternae of the Golgi apparatus [published eeratum appears in J Biol Chem 1989 Mar 5;264(7):4264]. J Biol Chem. 1989 Jan 5;264(1):646–653. [PubMed] [Google Scholar]
- Gonatas J., Stieber A., Olsnes S., Gonatas N. K. Pathways involved in fluid phase and adsorptive endocytosis in neuroblastoma. J Cell Biol. 1980 Dec;87(3 Pt 1):579–588. doi: 10.1083/jcb.87.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonatas N. K., Kim S. U., Stieber A., Avrameas S. Internalization of lectins in neuronal GERL. J Cell Biol. 1977 Apr;73(1):1–13. doi: 10.1083/jcb.73.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonatas N. K. Presidential address. The role of neuronal golgi apparatus in a centripetal membrane vesicular traffic. J Neuropathol Exp Neurol. 1982 Jan;41(1):6–17. doi: 10.1097/00005072-198201000-00002. [DOI] [PubMed] [Google Scholar]
- Hays A. P., Roxas A., Sadiq S. A., Vallejos H., D'Agati V., Thomas F. P., Torres R., Sherman W. H., Bailey-Braxton D., Hays A. G. A monoclonal IgA in a patient with amyotrophic lateral sclerosis reacts with neurofilaments and surface antigen on neuroblastoma cells. J Neuropathol Exp Neurol. 1990 Jul;49(4):383–398. doi: 10.1097/00005072-199007000-00003. [DOI] [PubMed] [Google Scholar]
- Lee V. M., Carden M. J., Schlaepfer W. W., Trojanowski J. Q. Monoclonal antibodies distinguish several differentially phosphorylated states of the two largest rat neurofilament subunits (NF-H and NF-M) and demonstrate their existence in the normal nervous system of adult rats. J Neurosci. 1987 Nov;7(11):3474–3488. doi: 10.1523/JNEUROSCI.07-11-03474.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morré D. M. Role of the Golgi apparatus in cellular pathology. J Electron Microsc Tech. 1991 Feb;17(2):200–211. doi: 10.1002/jemt.1060170207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourelatos Z., Adler H., Hirano A., Donnenfeld H., Gonatas J. O., Gonatas N. K. Fragmentation of the Golgi apparatus of motor neurons in amyotrophic lateral sclerosis revealed by organelle-specific antibodies. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4393–4395. doi: 10.1073/pnas.87.11.4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry T. L., Krieger C., Hansen S., Eisen A. Amyotrophic lateral sclerosis: amino acid levels in plasma and cerebrospinal fluid. Ann Neurol. 1990 Jul;28(1):12–17. doi: 10.1002/ana.410280105. [DOI] [PubMed] [Google Scholar]
- Plaitakis A. Glutamate dysfunction and selective motor neuron degeneration in amyotrophic lateral sclerosis: a hypothesis. Ann Neurol. 1990 Jul;28(1):3–8. doi: 10.1002/ana.410280103. [DOI] [PubMed] [Google Scholar]
- ROBBINS E., GONATAS N. K. HISTOCHEMICAL AND ULTRASTRUCTURAL STUDIES ON HELA CELL CULTURES EXPOSED TO SPINDLE INHIBITORS WITH SPECIAL REFERENCE TO THE INTERPHASE CELL. J Histochem Cytochem. 1964 Sep;12:704–711. doi: 10.1177/12.9.704. [DOI] [PubMed] [Google Scholar]
- Rothstein J. D., Tsai G., Kuncl R. W., Clawson L., Cornblath D. R., Drachman D. B., Pestronk A., Stauch B. L., Coyle J. T. Abnormal excitatory amino acid metabolism in amyotrophic lateral sclerosis. Ann Neurol. 1990 Jul;28(1):18–25. doi: 10.1002/ana.410280106. [DOI] [PubMed] [Google Scholar]
- Schmidt M. L., Carden M. J., Lee V. M., Trojanowski J. Q. Phosphate dependent and independent neurofilament epitopes in the axonal swellings of patients with motor neuron disease and controls. Lab Invest. 1987 Mar;56(3):282–294. [PubMed] [Google Scholar]
- Tandan R., Bradley W. G. Amyotrophic lateral sclerosis: Part 2. Etiopathogenesis. Ann Neurol. 1985 Oct;18(4):419–431. doi: 10.1002/ana.410180402. [DOI] [PubMed] [Google Scholar]
- Turner J. R., Tartakoff A. M. The response of the Golgi complex to microtubule alterations: the roles of metabolic energy and membrane traffic in Golgi complex organization. J Cell Biol. 1989 Nov;109(5):2081–2088. doi: 10.1083/jcb.109.5.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]