Abstract
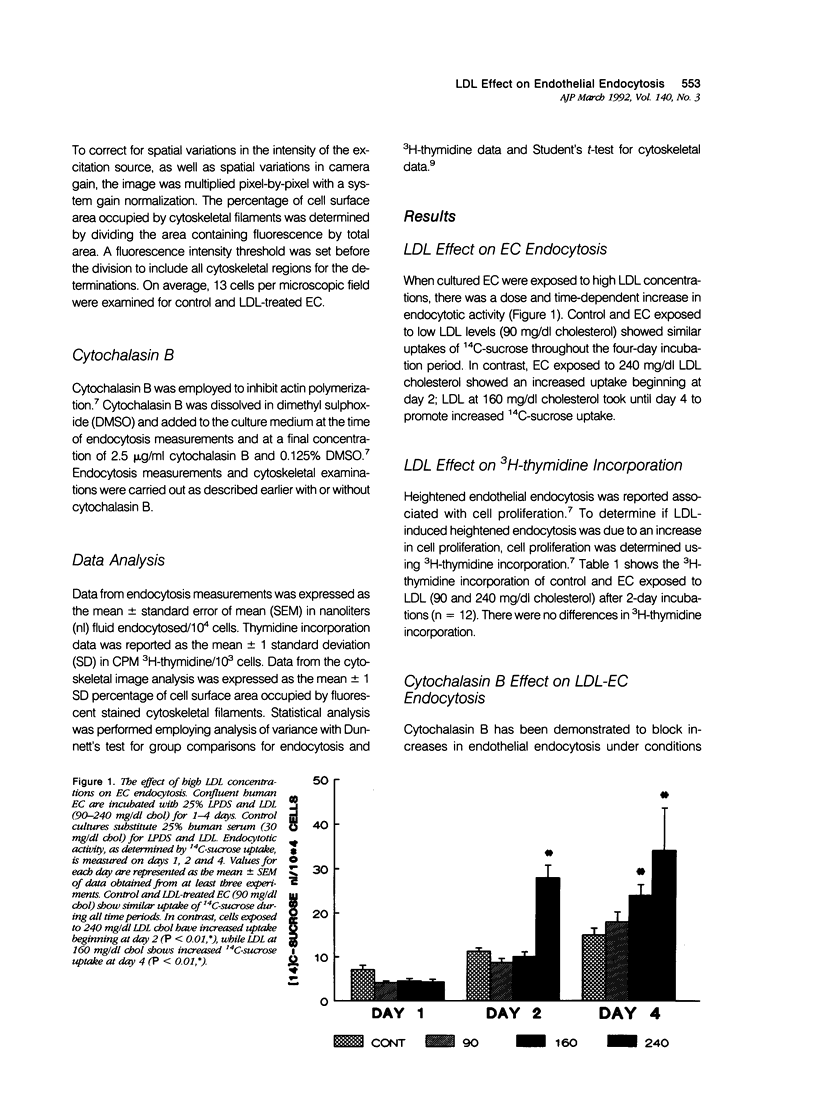
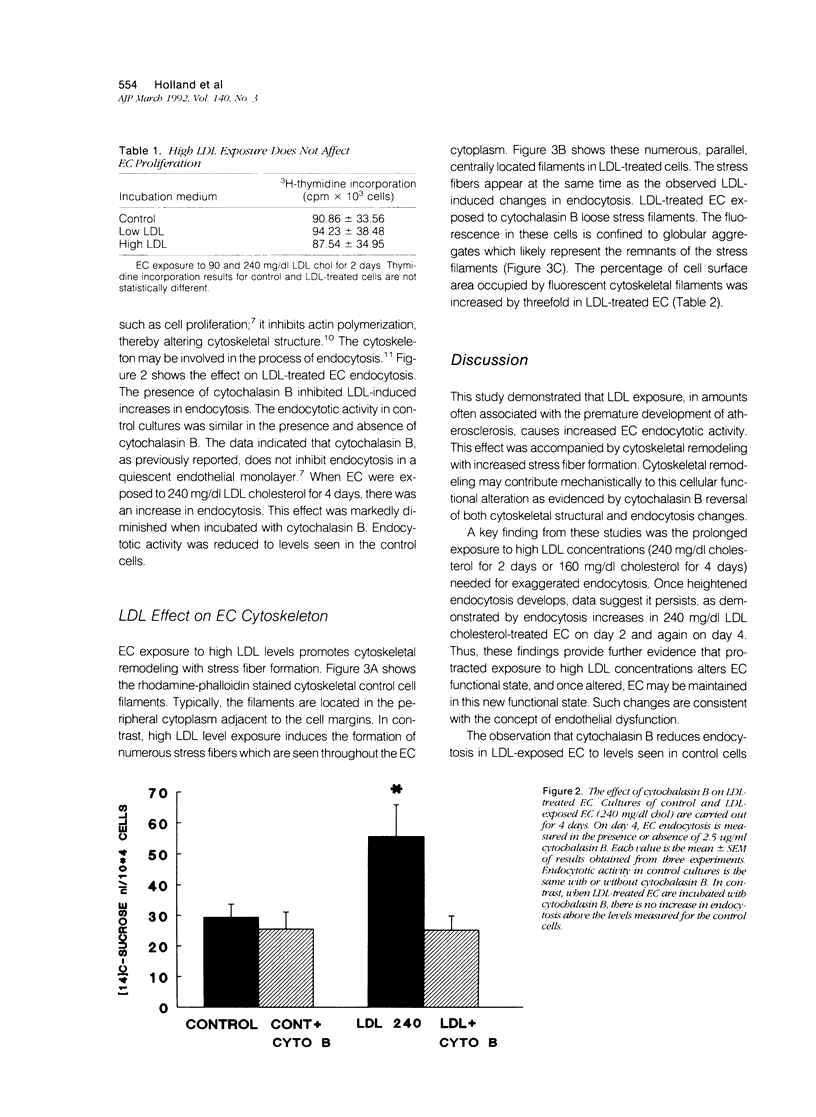
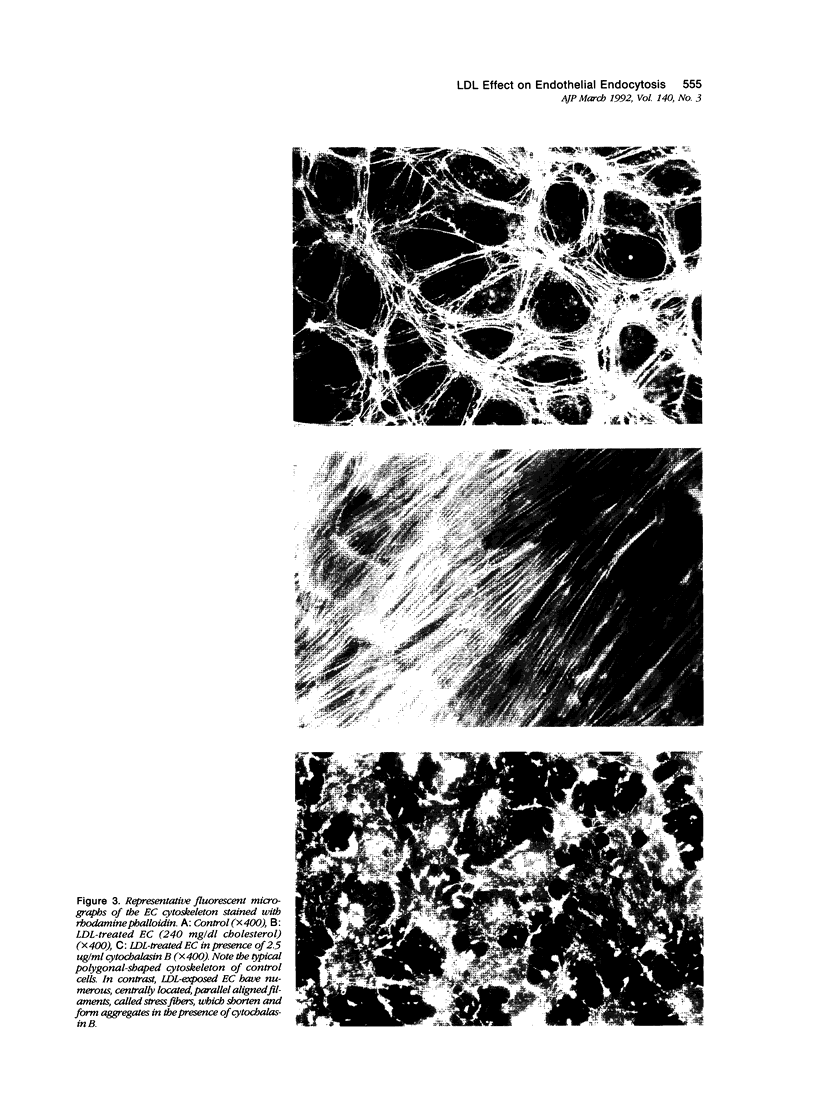
Cultured human umbilical vein endothelial cells (EC) exposed to atherogenic low-density lipoprotein (LDL) levels for protracted periods demonstrated heightened endocytosis. Confluent EC were incubated with LDL 90 to 240 mg/dl cholesterol for 1 to 4 days and endocytosis was measured by 14C-sucrose uptake. Control EC and cells incubated with 90 mg/dl LDL cholesterol showed similar uptakes of 14C-sucrose during all measured time periods. In contrast, EC exposed to 240 mg/dl LDL cholesterol showed an increase in endocytosis beginning at 2 days, whereas 160 mg/dl LDL cholesterol promoted increased uptake by 4 days. The endocytotic activity of LDL-perturbed EC is reduced to levels seen in control cells by cytochalasin B, an actin polymerization inhibitor. This finding suggests a modulatory role for the cytoskeleton in endocytosis changes. Examination of LDL-perturbed EC cytoskeleton reveals structural remodeling resulting in a marked increase in stress fibers. Cytochalasin B exposure causes a loss of stress fibers with the formation of globular filamental aggregates. Such LDL-induced cellular functional changes may contribute mechanistically to endothelial dysfunction, which is widely held to be a major contributing factor in the pathogenesis of atherosclerosis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Davies P. F., Selden S. C., 3rd, Schwartz S. M. Enhanced rates of fluid pinocytosis during exponential growth and monolayer regeneration by cultured arterial endothelial cells. J Cell Physiol. 1980 Feb;102(2):119–127. doi: 10.1002/jcp.1041020204. [DOI] [PubMed] [Google Scholar]
- Flanagan M. D., Lin S. Cytochalasins block actin filament elongation by binding to high affinity sites associated with F-actin. J Biol Chem. 1980 Feb 10;255(3):835–838. [PubMed] [Google Scholar]
- Guyton J. R., Shaffer D. R., Henry P. D. Stress fibers in endothelial cells overlying atherosclerotic lesions in rabbit aorta. Am J Med Sci. 1989 Aug;298(2):79–82. doi: 10.1097/00000441-198908000-00002. [DOI] [PubMed] [Google Scholar]
- Haberland M. E., Fong D., Cheng L. Malondialdehyde-altered protein occurs in atheroma of Watanabe heritable hyperlipidemic rabbits. Science. 1988 Jul 8;241(4862):215–218. doi: 10.1126/science.2455346. [DOI] [PubMed] [Google Scholar]
- Holland J. A., Pritchard K. A., Rogers N. J., Stemerman M. B. Perturbation of cultured human endothelial cells by atherogenic levels of low density lipoprotein. Am J Pathol. 1988 Sep;132(3):474–478. [PMC free article] [PubMed] [Google Scholar]
- Lin S. J., Jan K. M., Schuessler G., Weinbaum S., Chien S. Enhanced macromolecular permeability of aortic endothelial cells in association with mitosis. Atherosclerosis. 1988 Oct;73(2-3):223–232. doi: 10.1016/0021-9150(88)90045-7. [DOI] [PubMed] [Google Scholar]
- Morel D. W., DiCorleto P. E., Chisolm G. M. Endothelial and smooth muscle cells alter low density lipoprotein in vitro by free radical oxidation. Arteriosclerosis. 1984 Jul-Aug;4(4):357–364. doi: 10.1161/01.atv.4.4.357. [DOI] [PubMed] [Google Scholar]
- Morel D. W., Hessler J. R., Chisolm G. M. Low density lipoprotein cytotoxicity induced by free radical peroxidation of lipid. J Lipid Res. 1983 Aug;24(8):1070–1076. [PubMed] [Google Scholar]
- Nagelkerke J. F., Barto K. P., van Berkel T. J. In vivo and in vitro uptake and degradation of acetylated low density lipoprotein by rat liver endothelial, Kupffer, and parenchymal cells. J Biol Chem. 1983 Oct 25;258(20):12221–12227. [PubMed] [Google Scholar]
- Nagelkerke J. F., Havekes L., van Hinsbergh V. W., van Berkel T. J. In vivo catabolism of biologically modified LDL. Arteriosclerosis. 1984 May-Jun;4(3):256–264. doi: 10.1161/01.atv.4.3.256. [DOI] [PubMed] [Google Scholar]
- Phillips P. G., Tsan M. F. Hyperoxia causes increased albumin permeability of cultured endothelial monolayers. J Appl Physiol (1985) 1988 Mar;64(3):1196–1202. doi: 10.1152/jappl.1988.64.3.1196. [DOI] [PubMed] [Google Scholar]
- Pritchard K. A., Jr, Holland J. A., Rogers N. J., Crean C. C., Britton T. E., Onigman P., Stemerman M. B. Low-density lipoprotein preparation by combined diafiltration and ultracentrifugation. Anal Biochem. 1988 Oct;174(1):121–127. doi: 10.1016/0003-2697(88)90525-8. [DOI] [PubMed] [Google Scholar]
- Schwenke D. C., Carew T. E. Initiation of atherosclerotic lesions in cholesterol-fed rabbits. II. Selective retention of LDL vs. selective increases in LDL permeability in susceptible sites of arteries. Arteriosclerosis. 1989 Nov-Dec;9(6):908–918. doi: 10.1161/01.atv.9.6.908. [DOI] [PubMed] [Google Scholar]
- Singer I. I. Microfilament bundles and the control of pinocytotic vesicle distribution at the surfaces of normal and transformed fibroblasts. Exp Cell Res. 1979 Sep;122(2):251–264. doi: 10.1016/0014-4827(79)90302-1. [DOI] [PubMed] [Google Scholar]
- Steinberg D., Parthasarathy S., Carew T. E., Khoo J. C., Witztum J. L. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989 Apr 6;320(14):915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- Stemerman M. B., Morrel E. M., Burke K. R., Colton C. K., Smith K. A., Lees R. S. Local variation in arterial wall permeability to low density lipoprotein in normal rabbit aorta. Arteriosclerosis. 1986 Jan-Feb;6(1):64–69. doi: 10.1161/01.atv.6.1.64. [DOI] [PubMed] [Google Scholar]
- Vasile E., Simionescu M., Simionescu N. Visualization of the binding, endocytosis, and transcytosis of low-density lipoprotein in the arterial endothelium in situ. J Cell Biol. 1983 Jun;96(6):1677–1689. doi: 10.1083/jcb.96.6.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hinsbergh V. W., Scheffer M., Havekes L., Kempen H. J. Role of endothelial cells and their products in the modification of low-density lipoproteins. Biochim Biophys Acta. 1986 Aug 14;878(1):49–64. doi: 10.1016/0005-2760(86)90343-7. [DOI] [PubMed] [Google Scholar]



