Abstract
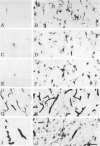
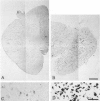
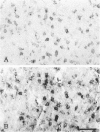
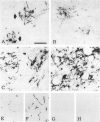
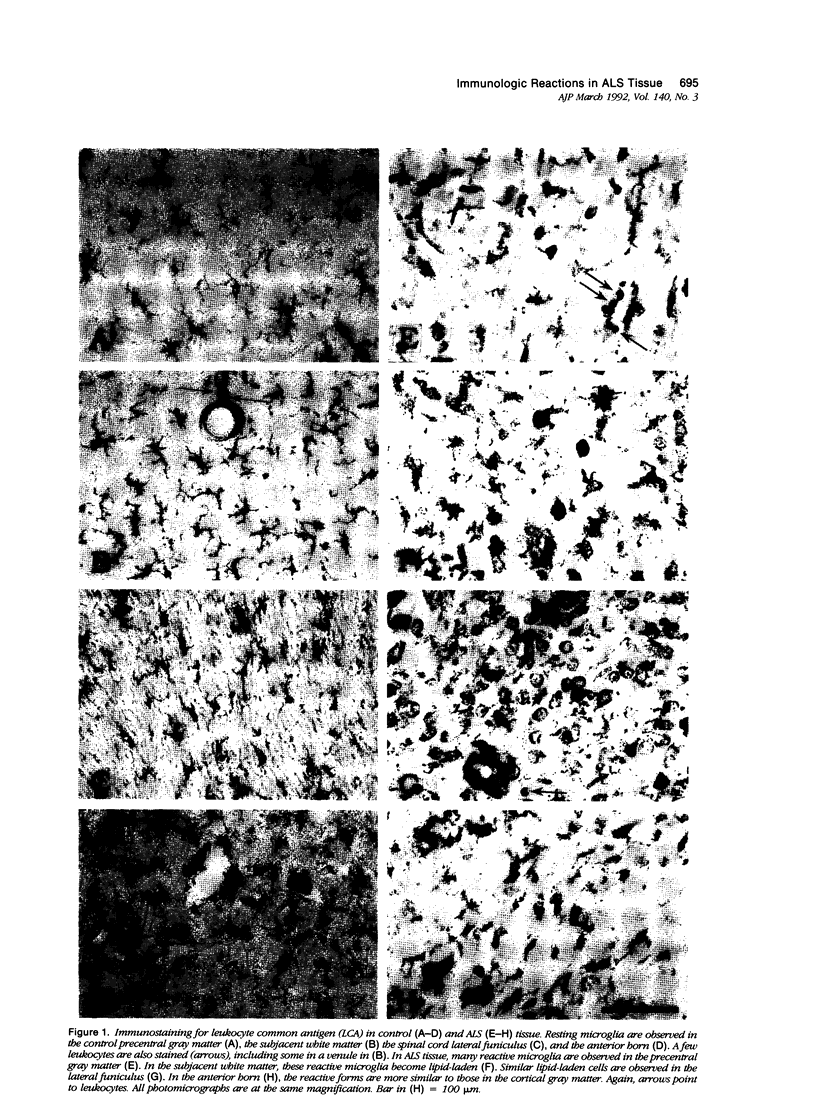
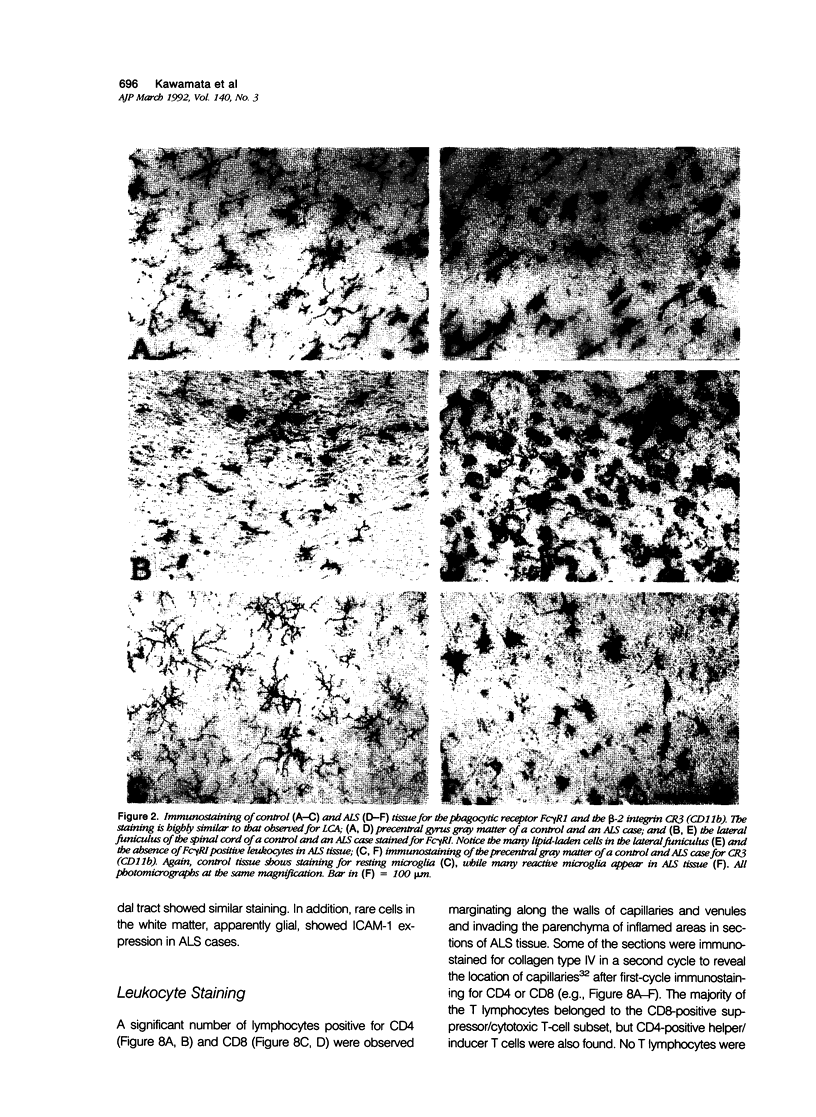
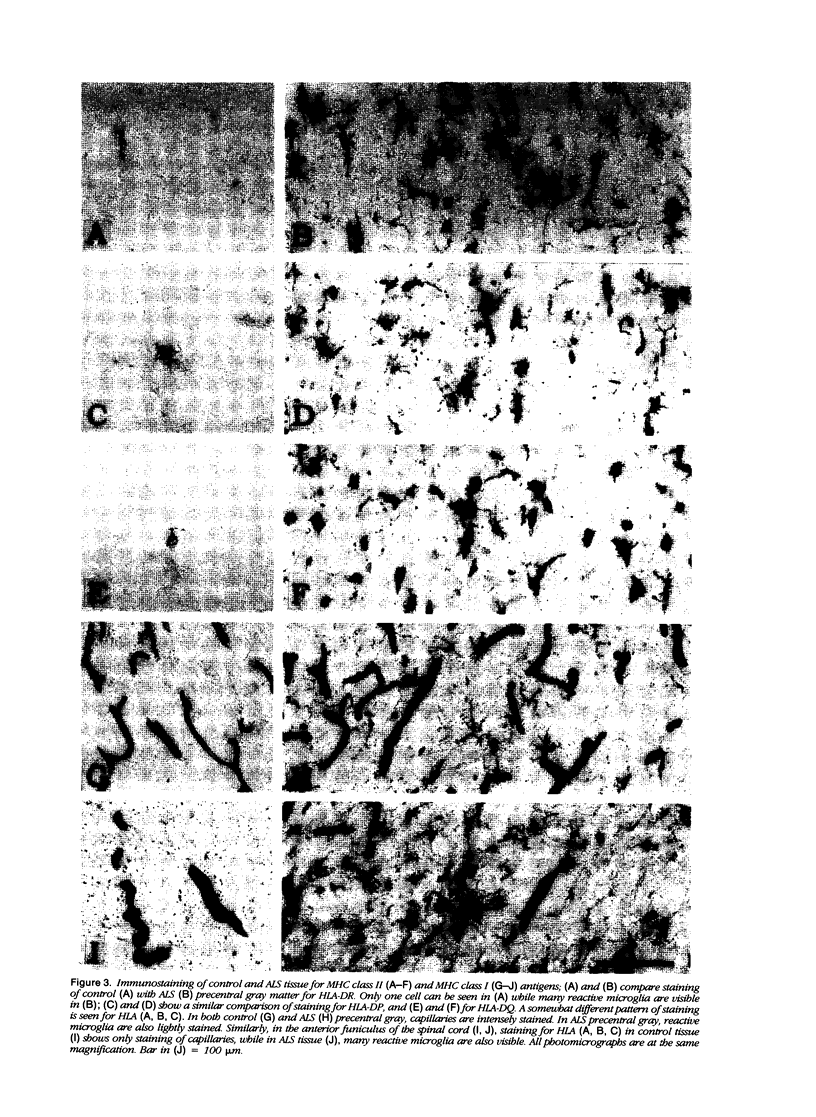
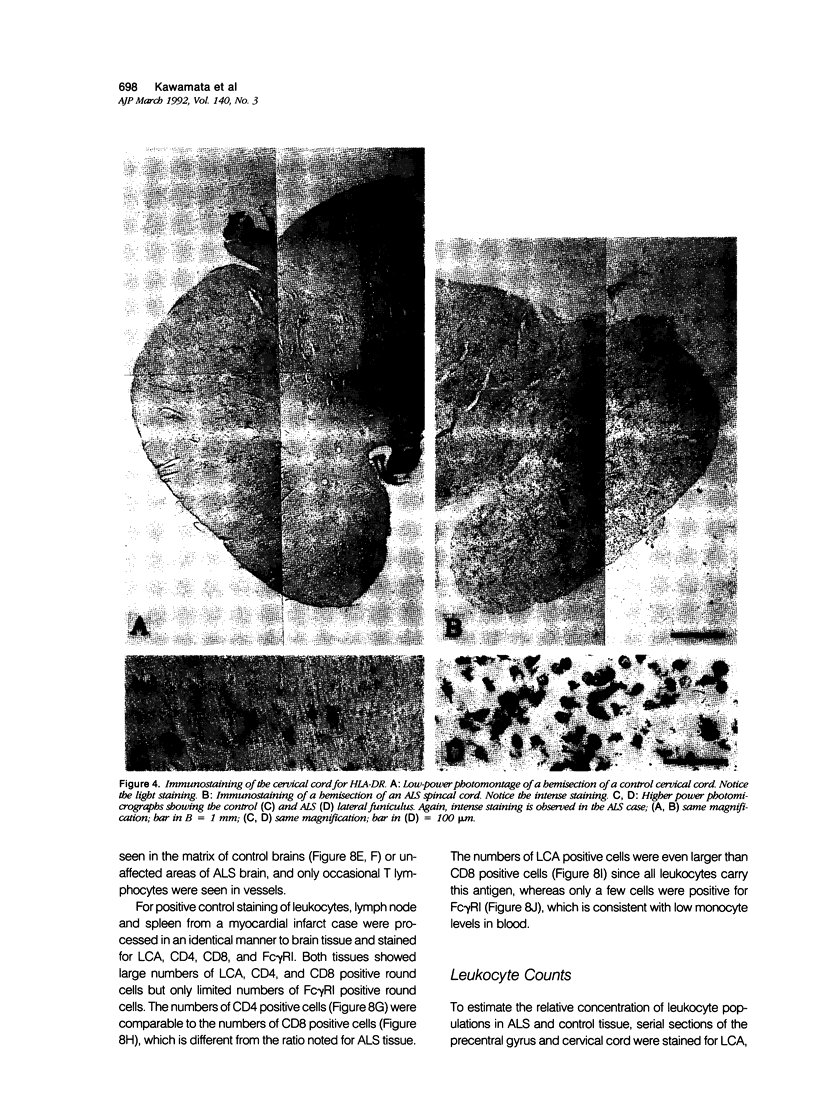
Expression of proteins associated with immune function was investigated immunohistochemically in postmortem brain and spinal cord of patients with amyotrophic lateral sclerosis (ALS). Reactive microglia/macrophages displaying high levels of leukocyte common antigen (LCA), the immunoglobulin receptor Fc gamma R1, lymphocyte function associated molecule-1 (LFA-1), the complement receptors CR3 and CR4, the class II major histocompatibility complex molecules HLA-DR, HLA-DP and HLA-DQ and common determinants of the class I HLA-A,B,C complex were abundant in affected areas in ALS. These areas included the primary motor cortex, motor nuclei of the brain stem, the anterior horn of the spinal cord, and the full extent of the corticospinal tract. A significant number of T lymphocytes of the helper/inducer (CD4+) and cytotoxic/suppressor (CD8+) subtypes were observed marginating along the walls of capillaries and venules and extending into the parenchyma of affected areas. Clusters of complement activated oligodendroglia as well as degenerating neurites positive for C3d and C4d were frequently detected in ALS-affected areas. These data provide evidence of immune-effector changes in ALS. They are consistent with an autoimmune or slow virus theory of the disorder, but may reflect only secondary changes.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama H., Itagaki S., McGeer P. L. Major histocompatibility complex antigen expression on rat microglia following epidural kainic acid lesions. J Neurosci Res. 1988;20(2):147–157. doi: 10.1002/jnr.490200202. [DOI] [PubMed] [Google Scholar]
- Akiyama H., McGeer P. L. Brain microglia constitutively express beta-2 integrins. J Neuroimmunol. 1990 Nov;30(1):81–93. doi: 10.1016/0165-5728(90)90055-r. [DOI] [PubMed] [Google Scholar]
- Boyle E. A., McGeer P. L. Cellular immune response in multiple sclerosis plaques. Am J Pathol. 1990 Sep;137(3):575–584. [PMC free article] [PubMed] [Google Scholar]
- Craggs R. I., Webster H. D. Ia antigens in the normal rat nervous system and in lesions of experimental allergic encephalomyelitis. Acta Neuropathol. 1985;68(4):263–272. doi: 10.1007/BF00690828. [DOI] [PubMed] [Google Scholar]
- Drachman D. B., Kuncl R. W. Amyotrophic lateral sclerosis: an unconventional autoimmune disease? Ann Neurol. 1989 Aug;26(2):269–274. doi: 10.1002/ana.410260214. [DOI] [PubMed] [Google Scholar]
- Engelhardt J. I., Appel S. H. IgG reactivity in the spinal cord and motor cortex in amyotrophic lateral sclerosis. Arch Neurol. 1990 Nov;47(11):1210–1216. doi: 10.1001/archneur.1990.00530110068019. [DOI] [PubMed] [Google Scholar]
- Fanger M. W., Shen L., Graziano R. F., Guyre P. M. Cytotoxicity mediated by human Fc receptors for IgG. Immunol Today. 1989 Mar;10(3):92–99. doi: 10.1016/0167-5699(89)90234-X. [DOI] [PubMed] [Google Scholar]
- Hays A. P., Roxas A., Sadiq S. A., Vallejos H., D'Agati V., Thomas F. P., Torres R., Sherman W. H., Bailey-Braxton D., Hays A. G. A monoclonal IgA in a patient with amyotrophic lateral sclerosis reacts with neurofilaments and surface antigen on neuroblastoma cells. J Neuropathol Exp Neurol. 1990 Jul;49(4):383–398. doi: 10.1097/00005072-199007000-00003. [DOI] [PubMed] [Google Scholar]
- Inuzuka T., Sato S., Yanagisawa K., Miyatake T. Antibodies to human spinal cord proteins in sera from patients with motor neuron disease and other neurological diseases. Eur Neurol. 1989;29(6):328–332. doi: 10.1159/000116440. [DOI] [PubMed] [Google Scholar]
- Itagaki S., McGeer P. L., Akiyama H. Presence of T-cytotoxic suppressor and leucocyte common antigen positive cells in Alzheimer's disease brain tissue. Neurosci Lett. 1988 Sep 12;91(3):259–264. doi: 10.1016/0304-3940(88)90690-8. [DOI] [PubMed] [Google Scholar]
- Kishimoto T. K., O'Connor K., Lee A., Roberts T. M., Springer T. A. Cloning of the beta subunit of the leukocyte adhesion proteins: homology to an extracellular matrix receptor defines a novel supergene family. Cell. 1987 Feb 27;48(4):681–690. doi: 10.1016/0092-8674(87)90246-7. [DOI] [PubMed] [Google Scholar]
- Lampson L. A., Kushner P. D., Sobel R. A. Major histocompatibility complex antigen expression in the affected tissues in amyotrophic lateral sclerosis. Ann Neurol. 1990 Sep;28(3):365–372. doi: 10.1002/ana.410280311. [DOI] [PubMed] [Google Scholar]
- Latov N., Hays A. P., Donofrio P. D., Liao J., Ito H., McGinnis S., Konstadoulakis M., Freddo L., Shy M. E., Manoussos K. Monoclonal IgM with unique specificity to gangliosides GM1 and GD1b and to lacto-N-tetraose associated with human motor neuron disease. Neurology. 1988 May;38(5):763–768. doi: 10.1212/wnl.38.5.763. [DOI] [PubMed] [Google Scholar]
- Marlin S. D., Springer T. A. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1). Cell. 1987 Dec 4;51(5):813–819. doi: 10.1016/0092-8674(87)90104-8. [DOI] [PubMed] [Google Scholar]
- McGeer P. L., Akiyama H., Itagaki S., McGeer E. G. Immune system response in Alzheimer's disease. Can J Neurol Sci. 1989 Nov;16(4 Suppl):516–527. doi: 10.1017/s0317167100029863. [DOI] [PubMed] [Google Scholar]
- McGeer P. L., Itagaki S., McGeer E. G. Expression of the histocompatibility glycoprotein HLA-DR in neurological disease. Acta Neuropathol. 1988;76(6):550–557. doi: 10.1007/BF00689592. [DOI] [PubMed] [Google Scholar]
- McGeer P. L., Zhu S. G., Dedhar S. Immunostaining of human brain capillaries by antibodies to very late antigens. J Neuroimmunol. 1990 Mar;26(3):213–218. doi: 10.1016/0165-5728(90)90003-6. [DOI] [PubMed] [Google Scholar]
- Micklem K. J., Sim R. B. Isolation of complement-fragment-iC3b-binding proteins by affinity chromatography. The identification of p150,95 as an iC3b-binding protein. Biochem J. 1985 Oct 1;231(1):233–236. doi: 10.1042/bj2310233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myones B. L., Dalzell J. G., Hogg N., Ross G. D. Neutrophil and monocyte cell surface p150,95 has iC3b-receptor (CR4) activity resembling CR3. J Clin Invest. 1988 Aug;82(2):640–651. doi: 10.1172/JCI113643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobile-Orazio E., Carpo M., Legname G., Meucci N., Sonnino S., Scarlato G. Anti-GM1 IgM antibodies in motor neuron disease and neuropathy. Neurology. 1990 Nov;40(11):1747–1750. doi: 10.1212/wnl.40.11.1747. [DOI] [PubMed] [Google Scholar]
- Patten B. M., Engel W. K. Phosphate and parathyroid disorders associated with the syndrome of amyotrophic lateral sclerosis. Adv Neurol. 1982;36:181–200. [PubMed] [Google Scholar]
- Penfield W. Microglia and the Process of Phagocytosis in Gliomas. Am J Pathol. 1925 Jan;1(1):77–90.15. [PMC free article] [PubMed] [Google Scholar]
- Pestronk A., Adams R. N., Cornblath D., Kuncl R. W., Drachman D. B., Clawson L. Patterns of serum IgM antibodies to GM1 and GD1a gangliosides in amyotrophic lateral sclerosis. Ann Neurol. 1989 Jan;25(1):98–102. doi: 10.1002/ana.410250118. [DOI] [PubMed] [Google Scholar]
- Roelofs-Iverson R. A., Mulder D. W., Elveback L. R., Kurland L. T., Molgaard C. A. ALS and heavy metals: a pilot case-control study. Neurology. 1984 Mar;34(3):393–395. doi: 10.1212/wnl.34.3.393. [DOI] [PubMed] [Google Scholar]
- Salazar-Grueso E. F., Routbort M. J., Martin J., Dawson G., Roos R. P. Polyclonal IgM anti-GM1 ganglioside antibody in patients with motor neuron disease and variants. Ann Neurol. 1990 May;27(5):558–563. doi: 10.1002/ana.410270517. [DOI] [PubMed] [Google Scholar]
- Scolding N. J., Morgan B. P., Houston A., Campbell A. K., Linington C., Compston D. A. Normal rat serum cytotoxicity against syngeneic oligodendrocytes. Complement activation and attack in the absence of anti-myelin antibodies. J Neurol Sci. 1989 Feb;89(2-3):289–300. doi: 10.1016/0022-510x(89)90030-0. [DOI] [PubMed] [Google Scholar]
- Shy M. E., Evans V. A., Lublin F. D., Knobler R. L., Heiman-Patterson T., Tahmoush A. J., Parry G., Schick P., DeRyk T. G. Antibodies to GM1 and GD1b in patients with motor neuron disease without plasma cell dyscrasia. Ann Neurol. 1989 May;25(5):511–513. doi: 10.1002/ana.410250517. [DOI] [PubMed] [Google Scholar]
- Streit W. J., Graeber M. B., Kreutzberg G. W. Expression of Ia antigen on perivascular and microglial cells after sublethal and lethal motor neuron injury. Exp Neurol. 1989 Aug;105(2):115–126. doi: 10.1016/0014-4886(89)90111-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandan R., Bradley W. G. Amyotrophic lateral sclerosis: Part 2. Etiopathogenesis. Ann Neurol. 1985 Oct;18(4):419–431. doi: 10.1002/ana.410180402. [DOI] [PubMed] [Google Scholar]
- Thomas F. P., Lee A. M., Romas S. N., Latov N. Monoclonal IgMs with anti-Gal(beta 1-3) GalNAc activity in lower motor neuron disease; identification of glycoprotein antigens in neural tissue and cross-reactivity with serum immunoglobulins. J Neuroimmunol. 1989 Jul;23(2):167–174. doi: 10.1016/0165-5728(89)90036-2. [DOI] [PubMed] [Google Scholar]
- Tooyama I., Kimura H., Akiyama H., McGeer P. L. Reactive microglia express class I and class II major histocompatibility complex antigens in Alzheimer's disease. Brain Res. 1990 Jul 23;523(2):273–280. doi: 10.1016/0006-8993(90)91496-4. [DOI] [PubMed] [Google Scholar]
- Troost D., Van den Oord J. J., Vianney de Jong J. M. Immunohistochemical characterization of the inflammatory infiltrate in amyotrophic lateral sclerosis. Neuropathol Appl Neurobiol. 1990 Oct;16(5):401–410. doi: 10.1111/j.1365-2990.1990.tb01276.x. [DOI] [PubMed] [Google Scholar]
- Whitehouse P. J., Wamsley J. K., Zarbin M. A., Price D. L., Tourtellotte W. W., Kuhar M. J. Amyotrophic lateral sclerosis: alterations in neurotransmitter receptors. Ann Neurol. 1983 Jul;14(1):8–16. doi: 10.1002/ana.410140103. [DOI] [PubMed] [Google Scholar]
- Yamada T., Akiyama H., McGeer P. L. Complement-activated oligodendroglia: a new pathogenic entity identified by immunostaining with antibodies to human complement proteins C3d and C4d. Neurosci Lett. 1990 May 4;112(2-3):161–166. doi: 10.1016/0304-3940(90)90196-g. [DOI] [PubMed] [Google Scholar]
- Younger D. S., Rowland L. P., Latov N., Sherman W., Pesce M., Lange D. J., Trojaborg W., Miller J. R., Lovelace R. E., Hays A. P. Motor neuron disease and amyotrophic lateral sclerosis: relation of high CSF protein content to paraproteinemia and clinical syndromes. Neurology. 1990 Apr;40(4):595–599. doi: 10.1212/wnl.40.4.595. [DOI] [PubMed] [Google Scholar]