Abstract
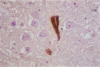
Membrane and cytoskeletal structures are known targets of oxidative injury. Brains from patients with Alzheimer's disease have cytoskeletal abnormalities and platelet and possible neuronal membrane lesions. The authors have recently demonstrated that superoxide anion is a powerful inducer of heat-shock protein synthesis, and have also shown that in response to oxidative stress or hyperthermia, intracellular levels of antioxidant enzymes increase to several folds. Whether the aforementioned mechanisms play a role in Alzheimer's disease has been suggested but is not totally established. While exploring this possibility, tissue sections from five brains with Alzheimer's disease and five neuropathologically normal age-matched controls were immunostained with polyclonal antibodies against superoxide dismutase (CuZn- and Mn- forms) and catalase. A standard avidin-biotin-peroxidase method was used for antigen detection. A subgroup of neurofibrillary tangles (15-25%) and senile plaques (50%) showed immunoreactivity for both enzymes with a staining pattern similar (but not identical) to that usually observed with antibodies against ubiquitin. Senile plaques displayed a granular pattern of immunostaining. Amyloid cores in mature classical plaques remained unstained. In addition, occasional elements with features consistent with reactive glial cells were strongly immunostained. Tangle-free neurons in both diseased and control brains showed weak to absent intracytoplasmic immunoreactivity. The immunoreactivity was totally abolished by preincubation of the primary antibodies with the corresponding purified antigens. These findings support the hypothesis that oxidative stress may be involved in the pathogenesis of Alzheimer's disease.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ananthan J., Goldberg A. L., Voellmy R. Abnormal proteins serve as eukaryotic stress signals and trigger the activation of heat shock genes. Science. 1986 Apr 25;232(4749):522–524. doi: 10.1126/science.3083508. [DOI] [PubMed] [Google Scholar]
- Ashburner M., Bonner J. J. The induction of gene activity in drosophilia by heat shock. Cell. 1979 Jun;17(2):241–254. doi: 10.1016/0092-8674(79)90150-8. [DOI] [PubMed] [Google Scholar]
- Atkinson B. G., Blaker T. W., Tomlinson J., Dean R. L. Ferritin is a translationally regulated heat shock protein of avian reticulocytes. J Biol Chem. 1990 Aug 25;265(24):14156–14162. [PubMed] [Google Scholar]
- Becker J., Mezger V., Courgeon A. M., Best-Belpomme M. Hydrogen peroxide activates immediate binding of a Drosophila factor to DNA heat-shock regulatory element in vivo and in vitro. Eur J Biochem. 1990 May 20;189(3):553–558. doi: 10.1111/j.1432-1033.1990.tb15522.x. [DOI] [PubMed] [Google Scholar]
- Blass J. P., Baker A. C., Ko L., Black R. S. Induction of Alzheimer antigens by an uncoupler of oxidative phosphorylation. Arch Neurol. 1990 Aug;47(8):864–869. doi: 10.1001/archneur.1990.00530080046009. [DOI] [PubMed] [Google Scholar]
- Borisy G. G., Marcum J. M., Olmsted J. B., Murphy D. B., Johnson K. A. Purification of tubulin and associated high molecular weight proteins from porcine brain and characterization of microtubule assembly in vitro. Ann N Y Acad Sci. 1975 Jun 30;253:107–132. doi: 10.1111/j.1749-6632.1975.tb19196.x. [DOI] [PubMed] [Google Scholar]
- Cohen M. L., Golde T. E., Usiak M. F., Younkin L. H., Younkin S. G. In situ hybridization of nucleus basalis neurons shows increased beta-amyloid mRNA in Alzheimer disease. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1227–1231. doi: 10.1073/pnas.85.4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton J. L., McCarthy B. J. Induction of the Drosophila heat shock response in isolated polytene nuclei. Cell. 1978 May;14(1):191–201. doi: 10.1016/0092-8674(78)90313-6. [DOI] [PubMed] [Google Scholar]
- Coss R. A., Dewey W. C., Bamburg J. R. Effects of hyperthermia on dividing Chinese hamster ovary cells and on microtubules in vitro. Cancer Res. 1982 Mar;42(3):1059–1071. [PubMed] [Google Scholar]
- Craig E. A. The heat shock response. CRC Crit Rev Biochem. 1985;18(3):239–280. doi: 10.3109/10409238509085135. [DOI] [PubMed] [Google Scholar]
- De Keyser J., Ebinger G., Vauquelin G. D1-dopamine receptor abnormality in frontal cortex points to a functional alteration of cortical cell membranes in Alzheimer's disease. Arch Neurol. 1990 Jul;47(7):761–763. doi: 10.1001/archneur.1990.00530070055011. [DOI] [PubMed] [Google Scholar]
- Delabar J. M., Goldgaber D., Lamour Y., Nicole A., Huret J. L., de Grouchy J., Brown P., Gajdusek D. C., Sinet P. M. Beta amyloid gene duplication in Alzheimer's disease and karyotypically normal Down syndrome. Science. 1987 Mar 13;235(4794):1390–1392. doi: 10.1126/science.2950593. [DOI] [PubMed] [Google Scholar]
- Evans P. H., Klinowski J., Yano E., Urano N. Alzheimer's disease: a pathogenic role for aluminosilicate-induced phagocytic free radicals. Free Radic Res Commun. 1989;6(5):317–321. doi: 10.3109/10715768909055157. [DOI] [PubMed] [Google Scholar]
- Glass J. R., DeWitt R. G., Cress A. E. Rapid loss of stress fibers in Chinese hamster ovary cells after hyperthermia. Cancer Res. 1985 Jan;45(1):258–262. [PubMed] [Google Scholar]
- Goldberg A. L., St John A. C. Intracellular protein degradation in mammalian and bacterial cells: Part 2. Annu Rev Biochem. 1976;45:747–803. doi: 10.1146/annurev.bi.45.070176.003531. [DOI] [PubMed] [Google Scholar]
- Hamos J. E., Oblas B., Pulaski-Salo D., Welch W. J., Bole D. G., Drachman D. A. Expression of heat shock proteins in Alzheimer's disease. Neurology. 1991 Mar;41(3):345–350. doi: 10.1212/wnl.41.3.345. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. The use of antiavidin antibody and avidin-biotin-peroxidase complex in immunoperoxidase technics. Am J Clin Pathol. 1981 Jun;75(6):816–821. doi: 10.1093/ajcp/75.6.816. [DOI] [PubMed] [Google Scholar]
- Huret J. L., Delabar J. M., Marlhens F., Aurias A., Nicole A., Berthier M., Tanzer J., Sinet P. M. Down syndrome with duplication of a region of chromosome 21 containing the CuZn superoxide dismutase gene without detectable karyotypic abnormality. Hum Genet. 1987 Mar;75(3):251–257. doi: 10.1007/BF00281069. [DOI] [PubMed] [Google Scholar]
- Iwamoto N., Suzuki Y., Makino Y., Haga C., Kosaka K., Iizuka R. Cell membrane changes in brains manifesting senile plaques: an immunohistochemical study of GM1 membranous ganglioside. Brain Res. 1990 Jul 2;522(1):152–156. doi: 10.1016/0006-8993(90)91592-5. [DOI] [PubMed] [Google Scholar]
- Khachaturian Z. S. Diagnosis of Alzheimer's disease. Arch Neurol. 1985 Nov;42(11):1097–1105. doi: 10.1001/archneur.1985.04060100083029. [DOI] [PubMed] [Google Scholar]
- La Fauci G., Lahiri D. K., Salton S. R., Robakis N. K. Characterization of the 5'-end region and the first two exons of the beta-protein precursor gene. Biochem Biophys Res Commun. 1989 Feb 28;159(1):297–304. doi: 10.1016/0006-291x(89)92437-6. [DOI] [PubMed] [Google Scholar]
- Levinson W., Oppermann H., Jackson J. Transition series metals and sulfhydryl reagents induce the synthesis of four proteins in eukaryotic cells. Biochim Biophys Acta. 1980;606(1):170–180. doi: 10.1016/0005-2787(80)90108-2. [DOI] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Lowe J., Blanchard A., Morrell K., Lennox G., Reynolds L., Billett M., Landon M., Mayer R. J. Ubiquitin is a common factor in intermediate filament inclusion bodies of diverse type in man, including those of Parkinson's disease, Pick's disease, and Alzheimer's disease, as well as Rosenthal fibres in cerebellar astrocytomas, cytoplasmic bodies in muscle, and mallory bodies in alcoholic liver disease. J Pathol. 1988 May;155(1):9–15. doi: 10.1002/path.1711550105. [DOI] [PubMed] [Google Scholar]
- Lowe J., Blanchard A., Morrell K., Lennox G., Reynolds L., Billett M., Landon M., Mayer R. J. Ubiquitin is a common factor in intermediate filament inclusion bodies of diverse type in man, including those of Parkinson's disease, Pick's disease, and Alzheimer's disease, as well as Rosenthal fibres in cerebellar astrocytomas, cytoplasmic bodies in muscle, and mallory bodies in alcoholic liver disease. J Pathol. 1988 May;155(1):9–15. doi: 10.1002/path.1711550105. [DOI] [PubMed] [Google Scholar]
- Marklund S. L., Adolfsson R., Gottfries C. G., Winblad B. Superoxide dismutase isoenzymes in normal brains and in brains from patients with dementia of Alzheimer type. J Neurol Sci. 1985 Mar;67(3):319–325. doi: 10.1016/0022-510x(85)90156-x. [DOI] [PubMed] [Google Scholar]
- Mori H., Kondo J., Ihara Y. Ubiquitin is a component of paired helical filaments in Alzheimer's disease. Science. 1987 Mar 27;235(4796):1641–1644. doi: 10.1126/science.3029875. [DOI] [PubMed] [Google Scholar]
- Neve R. L., Rogers J., Higgins G. A. The Alzheimer amyloid precursor-related transcript lacking the beta/A4 sequence is specifically increased in Alzheimer's disease brain. Neuron. 1990 Sep;5(3):329–338. doi: 10.1016/0896-6273(90)90169-g. [DOI] [PubMed] [Google Scholar]
- Omar R. A., Lanks K. W. Heat shock protein synthesis and cell survival in clones of normal and simian virus 40-transformed mouse embryo cells. Cancer Res. 1984 Sep;44(9):3976–3982. [PubMed] [Google Scholar]
- Omar R. A., Yano S., Kikkawa Y. Antioxidant enzymes and survival of normal and simian virus 40-transformed mouse embryo cells after hyperthermia. Cancer Res. 1987 Jul 1;47(13):3473–3476. [PubMed] [Google Scholar]
- Omar R., Pappolla M., Saran B. Immunocytochemical detection of the 70-kd heat shock protein in alcoholic liver disease. Arch Pathol Lab Med. 1990 Jun;114(6):589–592. [PubMed] [Google Scholar]
- Pappolla M. A., Alzofon J., McMahon J., Theodoropoulos T. J. Ultrastructural evidence that insoluble microtubules are components of the neurofibrillary tangle. Eur Arch Psychiatry Neurol Sci. 1990;239(5):314–319. doi: 10.1007/BF01735057. [DOI] [PubMed] [Google Scholar]
- Pappolla M. A., Omar R. A., Vinters H. V. Image analysis microspectroscopy shows that neurons participate in the genesis of a subset of early primitive (diffuse) senile plaques. Am J Pathol. 1991 Sep;139(3):599–607. [PMC free article] [PubMed] [Google Scholar]
- Pappolla M. A., Omar R., Saran B. The "normal" brain. "Abnormal" ubiquitinilated deposits highlight an age-related protein change. Am J Pathol. 1989 Oct;135(4):585–591. [PMC free article] [PubMed] [Google Scholar]
- Pappolla M. A., Omar R., Theodoropoulos T. J. Two distinct types of intraneuronal neurofibrillary tangles highlighted by polarized light after silver impregnation with a modified Bielschowsky method. Arch Pathol Lab Med. 1991 Feb;115(2):131–133. [PubMed] [Google Scholar]
- Parker W. D., Jr, Filley C. M., Parks J. K. Cytochrome oxidase deficiency in Alzheimer's disease. Neurology. 1990 Aug;40(8):1302–1303. doi: 10.1212/wnl.40.8.1302. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. Speculations on the functions of the major heat shock and glucose-regulated proteins. Cell. 1986 Sep 26;46(7):959–961. doi: 10.1016/0092-8674(86)90693-8. [DOI] [PubMed] [Google Scholar]
- Percy M. E., Dalton A. J., Markovic V. D., McLachlan D. R., Hummel J. T., Rusk A. C., Andrews D. F. Red cell superoxide dismutase, glutathione peroxidase and catalase in Down syndrome patients with and without manifestations of Alzheimer disease. Am J Med Genet. 1990 Apr;35(4):459–467. doi: 10.1002/ajmg.1320350403. [DOI] [PubMed] [Google Scholar]
- Perez N., Sugar J., Charya S., Johnson G., Merril C., Bierer L., Perl D., Haroutunian V., Wallace W. Increased synthesis and accumulation of heat shock 70 proteins in Alzheimer's disease. Brain Res Mol Brain Res. 1991 Oct;11(3-4):249–254. doi: 10.1016/0169-328x(91)90033-t. [DOI] [PubMed] [Google Scholar]
- Schlesinger M. J. Heat shock proteins: the search for functions. J Cell Biol. 1986 Aug;103(2):321–325. doi: 10.1083/jcb.103.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciandra J. J., Subjeck J. R., Hughes C. S. Induction of glucose-regulated proteins during anaerobic exposure and of heat-shock proteins after reoxygenation. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4843–4847. doi: 10.1073/pnas.81.15.4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. D., Carney J. M., Starke-Reed P. E., Oliver C. N., Stadtman E. R., Floyd R. A., Markesbery W. R. Excess brain protein oxidation and enzyme dysfunction in normal aging and in Alzheimer disease. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10540–10543. doi: 10.1073/pnas.88.23.10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville M. J., Percy M. E., Bergeron C., Yoong L. K., Grima E. A., McLachlan D. R. Localization and quantitation of 68 kDa neurofilament and superoxide dismutase-1 mRNA in Alzheimer brains. Brain Res Mol Brain Res. 1991 Jan;9(1-2):1–8. doi: 10.1016/0169-328x(91)90123-f. [DOI] [PubMed] [Google Scholar]
- Subbarao K. V., Richardson J. S., Ang L. C. Autopsy samples of Alzheimer's cortex show increased peroxidation in vitro. J Neurochem. 1990 Jul;55(1):342–345. doi: 10.1111/j.1471-4159.1990.tb08858.x. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Nakamura S., Ueda K., Kameyama M., Shiojiri S., Takahashi Y., Kitaguchi N., Ito H. Three types of amyloid protein precursor mRNA in human brain: their differential expression in Alzheimer's disease. Biochem Biophys Res Commun. 1988 Dec 15;157(2):472–479. doi: 10.1016/s0006-291x(88)80273-0. [DOI] [PubMed] [Google Scholar]
- Wiegant F. A., van Bergen en Henegouwen P. M., van Dongen G., Linnemans W. A. Stress-induced thermotolerance of the cytoskeleton of mouse neuroblastoma N2A cells and rat Reuber H35 hepatoma cells. Cancer Res. 1987 Mar 15;47(6):1674–1680. [PubMed] [Google Scholar]
- Wisniewski K. E., Dalton A. J., McLachlan C., Wen G. Y., Wisniewski H. M. Alzheimer's disease in Down's syndrome: clinicopathologic studies. Neurology. 1985 Jul;35(7):957–961. doi: 10.1212/wnl.35.7.957. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Hirano A. A comparative study of modified Bielschowsky, Bodian and thioflavin S stains on Alzheimer's neurofibrillary tangles. Neuropathol Appl Neurobiol. 1986 Jan-Feb;12(1):3–9. doi: 10.1111/j.1365-2990.1986.tb00677.x. [DOI] [PubMed] [Google Scholar]
- Zemlan F. P., Thienhaus O. J., Bosmann H. B. Superoxide dismutase activity in Alzheimer's disease: possible mechanism for paired helical filament formation. Brain Res. 1989 Jan 2;476(1):160–162. doi: 10.1016/0006-8993(89)91550-3. [DOI] [PubMed] [Google Scholar]
- Zubenko G. S., Cohen B. M., Boller F., Malinakova I., Keefe N., Chojnacki B. Platelet membrane abnormality in Alzheimer's disease. Ann Neurol. 1987 Aug;22(2):237–244. doi: 10.1002/ana.410220208. [DOI] [PubMed] [Google Scholar]
- Zubenko G. S., Cohen B. M., Reynolds C. F., 3rd, Boller F., Malinakova I., Keefe N. Platelet membrane fluidity in Alzheimer's disease and major depression. Am J Psychiatry. 1987 Jul;144(7):860–868. doi: 10.1176/ajp.144.7.860. [DOI] [PubMed] [Google Scholar]
- Zubenko G. S., Sauer P. SOD-1 activity and platelet membrane fluidity in Alzheimer's disease. Biol Psychiatry. 1989 Mar 15;25(6):671–678. doi: 10.1016/0006-3223(89)90236-9. [DOI] [PubMed] [Google Scholar]