Abstract
Activated lymphocytes and malignant lymphoma cells derived from them (Ki-1 positive lymphoma cells) share similar mechanisms of proliferation. To further examine the inhibitory role of endogenous transforming growth factor beta (TGF beta) in Ki-1 positive lymphoma cells, the authors studied anti-TGF beta antibodies and measured their effect on proliferation. A monoclonal antibody (T1A5) prepared against a unique antigenic epitope of high molecular weight Hodgkin's TGF beta and a polyclonal rabbit antibody prepared against highly purified 25,000 D porcine platelet TGF beta 1 were used. Both antibodies are shown here to inhibit the biological activity of Hodgkin's TGF beta and to crossreact with their respective antigens in immunoblotting. DNA synthesis by Ki-1 lymphoma cells was increased 138-fold by anti-TGF beta 1 antibody and 262-fold by anti-Hodgkin's TGF beta. Exogenous TGF beta 1 suppression was completely reversed by anti-TGF beta 1 antibody and IL-2-induced proliferation was markedly potentiated (41 fold). L-428 Reed-Sternberg cells secrete physiologically active TGF beta but have fewer than 500 TGF beta receptor sites per cell; no significant proliferative response was measured for either anti-TGF beta 1 or anti-Hodgkin's TGF beta. These results show the suppressive effect of exogenous TGF beta 1 on indolent Ki-1 lymphoma cells and suggest that the endogenous secretion of high molecular weight physiologically active TGF beta is important in maintaining the indolent nature of this low-grade Ki-1 positive lymphoma.
Full text
PDF

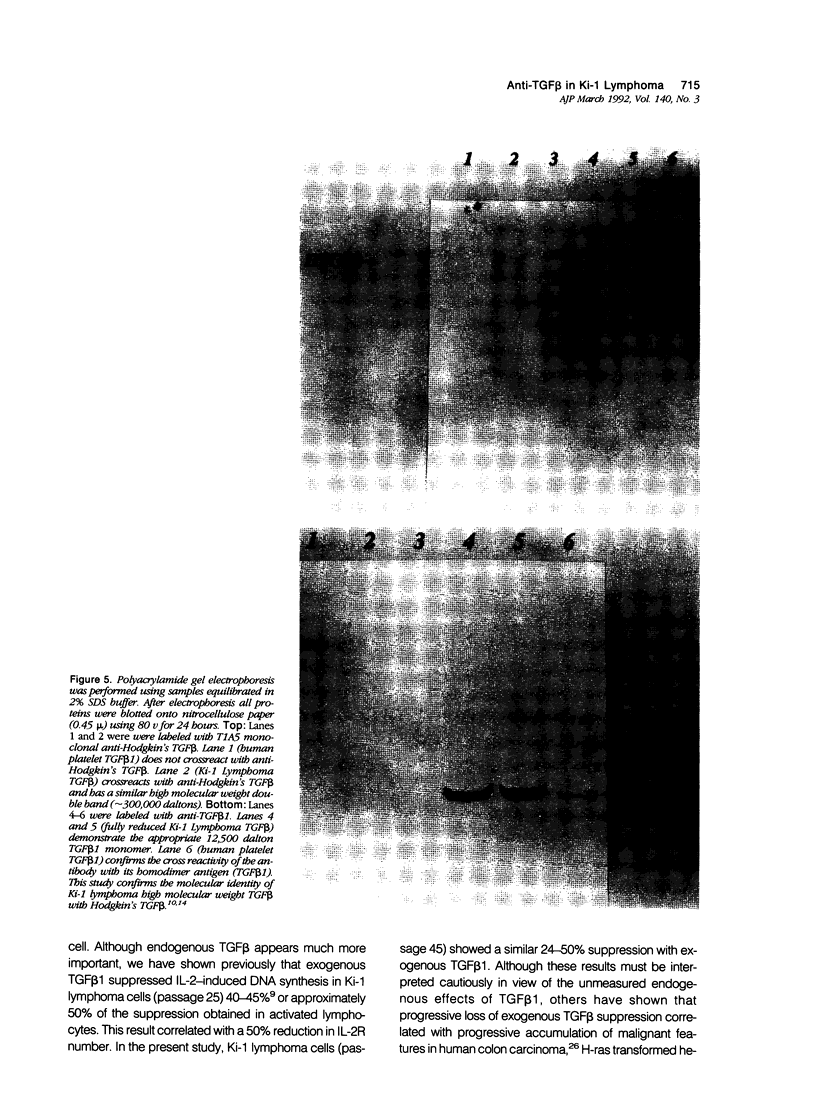



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agnarsson B. A., Kadin M. E. Ki-1 positive large cell lymphoma. A morphologic and immunologic study of 19 cases. Am J Surg Pathol. 1988 Apr;12(4):264–274. doi: 10.1097/00000478-198804000-00002. [DOI] [PubMed] [Google Scholar]
- Andreesen R., Osterholz J., Löhr G. W., Bross K. J. A Hodgkin cell-specific antigen is expressed on a subset of auto- and alloactivated T (helper) lymphoblasts. Blood. 1984 Jun;63(6):1299–1302. [PubMed] [Google Scholar]
- Antonelli-Orlidge A., Saunders K. B., Smith S. R., D'Amore P. A. An activated form of transforming growth factor beta is produced by cocultures of endothelial cells and pericytes. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4544–4548. doi: 10.1073/pnas.86.12.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryckaert M. C., Lindroth M., Lönn A., Tobelem G., Wasteson A. Transforming growth factor (TGF beta) decreases the proliferation of human bone marrow fibroblasts by inhibiting the platelet-derived growth factor (PDGF) binding. Exp Cell Res. 1988 Dec;179(2):311–321. doi: 10.1016/0014-4827(88)90270-4. [DOI] [PubMed] [Google Scholar]
- Coiffier B., Berger F., Bryon P. A., Magaud J. P. T-cell lymphomas: immunologic, histologic, clinical, and therapeutic analysis of 63 cases. J Clin Oncol. 1988 Oct;6(10):1584–1589. doi: 10.1200/JCO.1988.6.10.1584. [DOI] [PubMed] [Google Scholar]
- Danielpour D., Sporn M. B. Differential inhibition of transforming growth factor beta 1 and beta 2 activity by alpha 2-macroglobulin. J Biol Chem. 1990 Apr 25;265(12):6973–6977. [PubMed] [Google Scholar]
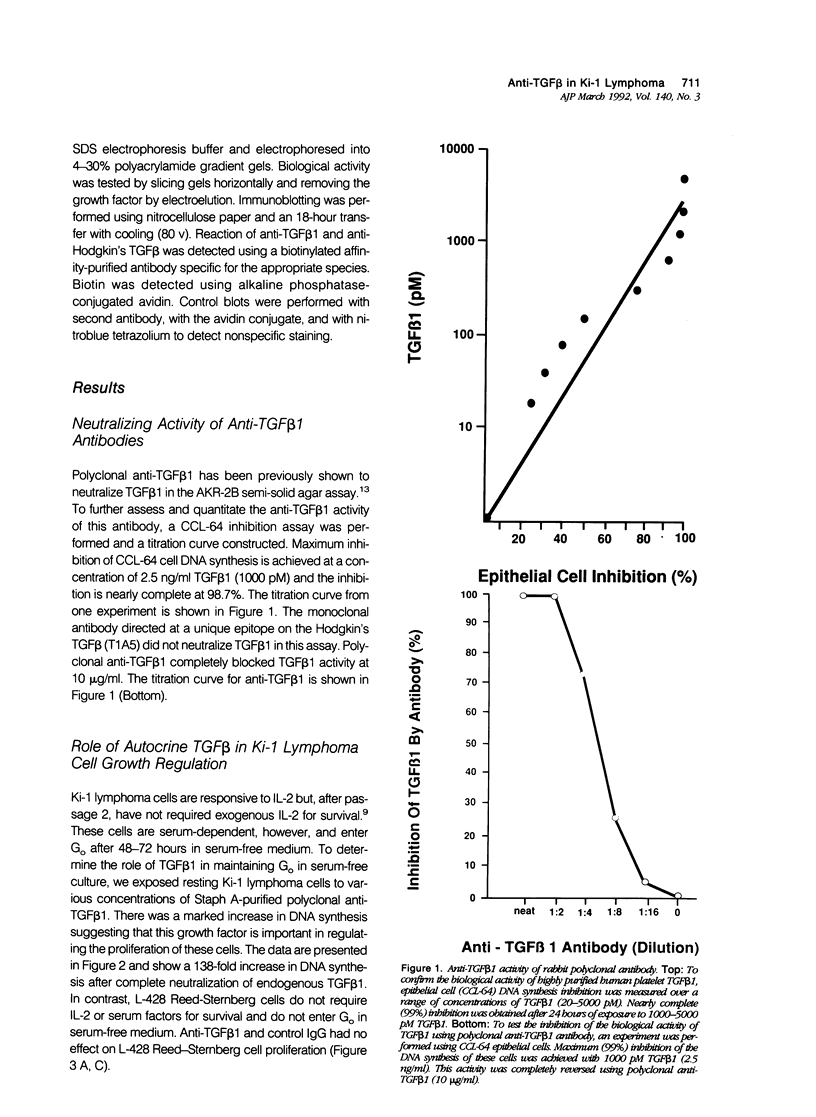
- Diehl V., Kirchner H. H., Burrichter H., Stein H., Fonatsch C., Gerdes J., Schaadt M., Heit W., Uchanska-Ziegler B., Ziegler A. Characteristics of Hodgkin's disease-derived cell lines. Cancer Treat Rep. 1982 Apr;66(4):615–632. [PubMed] [Google Scholar]
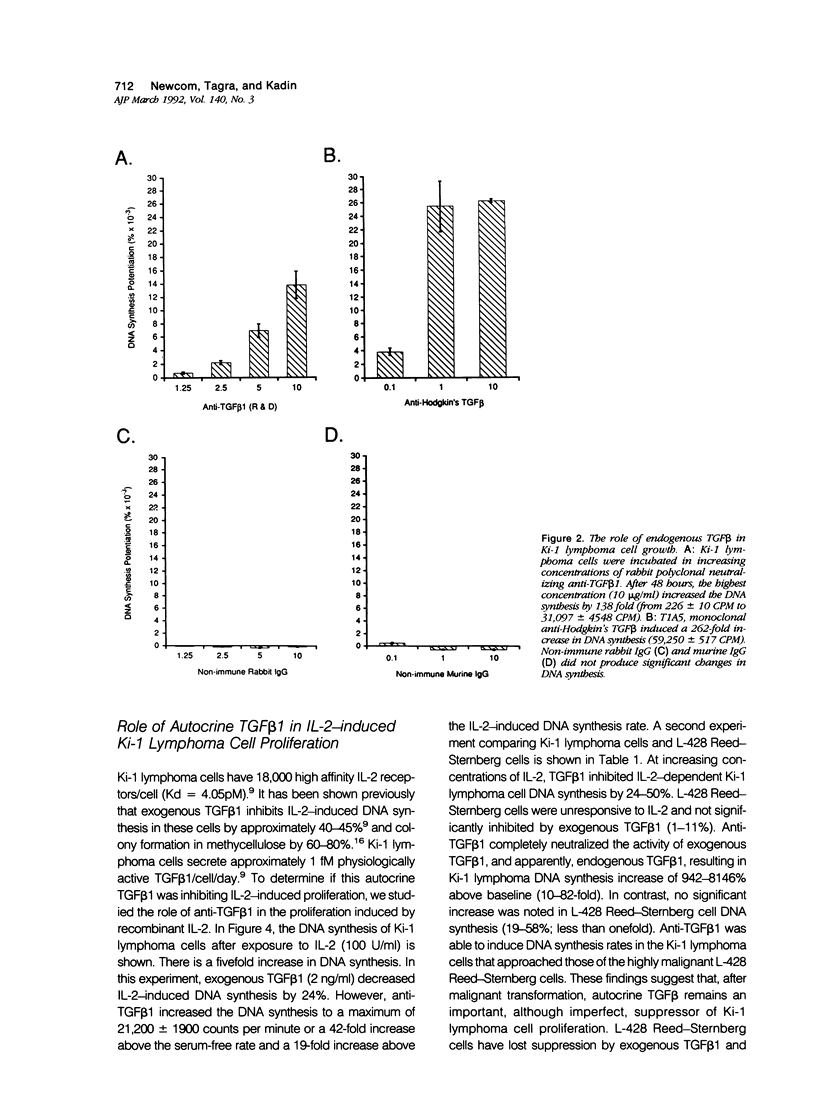
- Graycar J. L., Miller D. A., Arrick B. A., Lyons R. M., Moses H. L., Derynck R. Human transforming growth factor-beta 3: recombinant expression, purification, and biological activities in comparison with transforming growth factors-beta 1 and -beta 2. Mol Endocrinol. 1989 Dec;3(12):1977–1986. doi: 10.1210/mend-3-12-1977. [DOI] [PubMed] [Google Scholar]
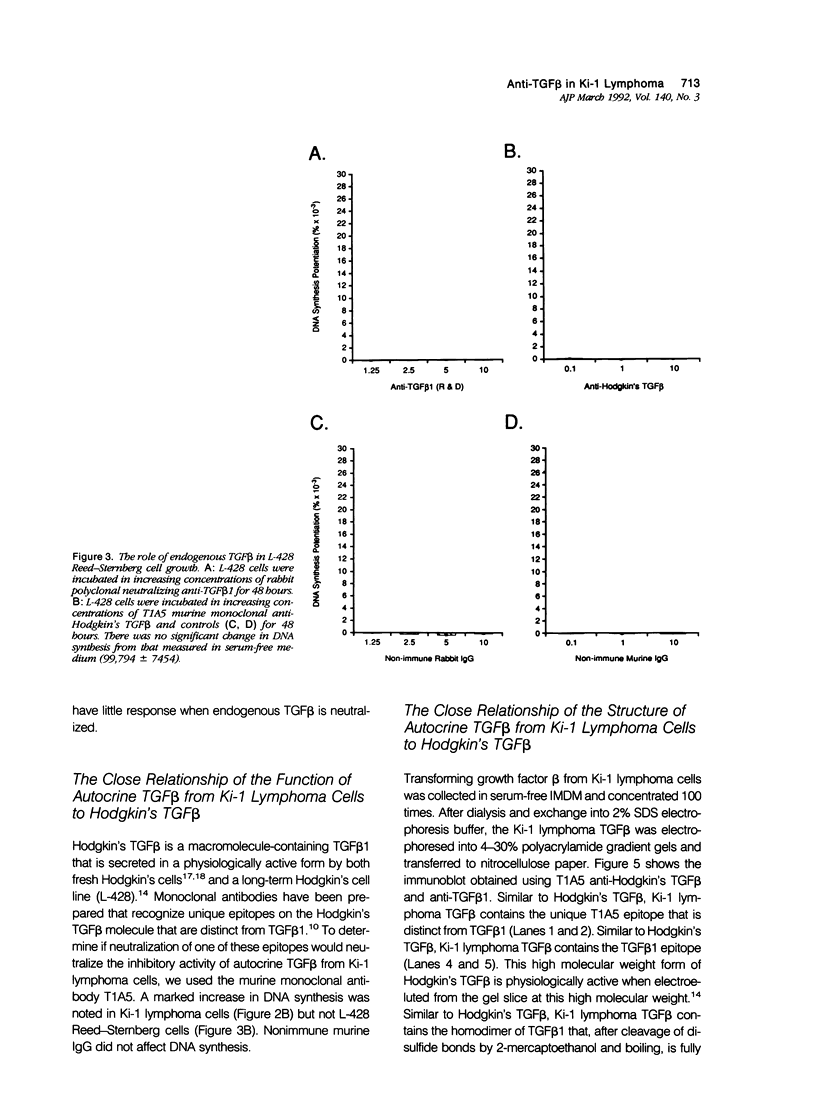
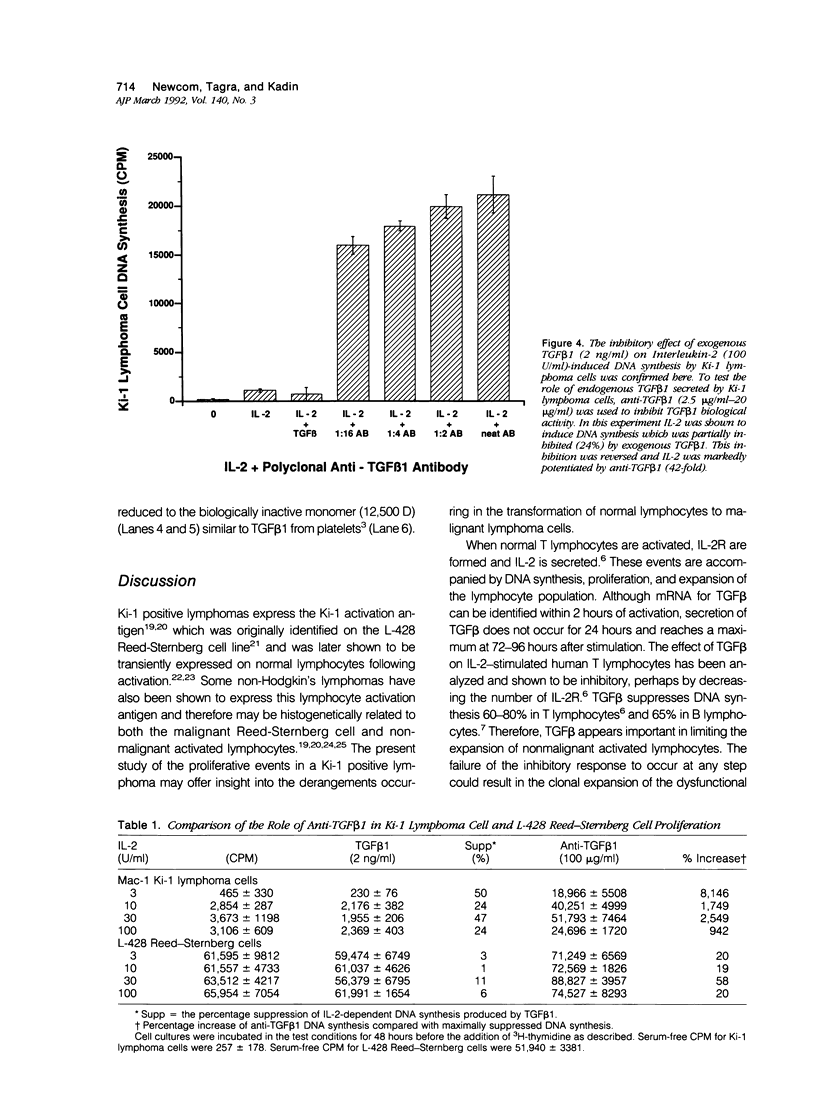
- Gronwald R. G., Seifert R. A., Bowen-Pope D. F. Differential regulation of expression of two platelet-derived growth factor receptor subunits by transforming growth factor-beta. J Biol Chem. 1989 May 15;264(14):8120–8125. [PubMed] [Google Scholar]
- Heino J., Ignotz R. A., Hemler M. E., Crouse C., Massagué J. Regulation of cell adhesion receptors by transforming growth factor-beta. Concomitant regulation of integrins that share a common beta 1 subunit. J Biol Chem. 1989 Jan 5;264(1):380–388. [PubMed] [Google Scholar]
- Hoosein N. M., McKnight M. K., Levine A. E., Mulder K. M., Childress K. E., Brattain D. E., Brattain M. G. Differential sensitivity of subclasses of human colon carcinoma cell lines to the growth inhibitory effects of transforming growth factor-beta 1. Exp Cell Res. 1989 Apr;181(2):442–453. doi: 10.1016/0014-4827(89)90101-8. [DOI] [PubMed] [Google Scholar]
- Houck K. A., Michalopoulos G. K., Strom S. C. Introduction of a Ha-ras oncogene into rat liver epithelial cells and parenchymal hepatocytes confers resistance to the growth inhibitory effects of TGF-beta. Oncogene. 1989 Jan;4(1):19–25. [PubMed] [Google Scholar]
- Hubbs A. F., Hahn F. F., Thomassen D. G. Increased resistance to transforming growth factor beta accompanies neoplastic progression of rat tracheal epithelial cells. Carcinogenesis. 1989 Sep;10(9):1599–1605. doi: 10.1093/carcin/10.9.1599. [DOI] [PubMed] [Google Scholar]
- Jullien P., Berg T. M., Lawrence D. A. Acidic cellular environments: activation of latent TGF-beta and sensitization of cellular responses to TGF-beta and EGF. Int J Cancer. 1989 May 15;43(5):886–891. doi: 10.1002/ijc.2910430525. [DOI] [PubMed] [Google Scholar]
- Kadin M. E. Common activated helper-T-cell origin for lymphomatoid papulosis, mycosis fungoides, and some types of Hodgkin's disease. Lancet. 1985 Oct 19;2(8460):864–865. doi: 10.1016/s0140-6736(85)90128-x. [DOI] [PubMed] [Google Scholar]
- Kadin M. E., Sako D., Berliner N., Franklin W., Woda B., Borowitz M., Ireland K., Schweid A., Herzog P., Lange B. Childhood Ki-1 lymphoma presenting with skin lesions and peripheral lymphadenopathy. Blood. 1986 Nov;68(5):1042–1049. [PubMed] [Google Scholar]
- Kanzaki T., Olofsson A., Morén A., Wernstedt C., Hellman U., Miyazono K., Claesson-Welsh L., Heldin C. H. TGF-beta 1 binding protein: a component of the large latent complex of TGF-beta 1 with multiple repeat sequences. Cell. 1990 Jun 15;61(6):1051–1061. doi: 10.1016/0092-8674(90)90069-q. [DOI] [PubMed] [Google Scholar]
- Kehrl J. H., Roberts A. B., Wakefield L. M., Jakowlew S., Sporn M. B., Fauci A. S. Transforming growth factor beta is an important immunomodulatory protein for human B lymphocytes. J Immunol. 1986 Dec 15;137(12):3855–3860. [PubMed] [Google Scholar]
- Kehrl J. H., Wakefield L. M., Roberts A. B., Jakowlew S., Alvarez-Mon M., Derynck R., Sporn M. B., Fauci A. S. Production of transforming growth factor beta by human T lymphocytes and its potential role in the regulation of T cell growth. J Exp Med. 1986 May 1;163(5):1037–1050. doi: 10.1084/jem.163.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr L. D., Miller D. B., Matrisian L. M. TGF-beta 1 inhibition of transin/stromelysin gene expression is mediated through a Fos binding sequence. Cell. 1990 Apr 20;61(2):267–278. doi: 10.1016/0092-8674(90)90807-q. [DOI] [PubMed] [Google Scholar]
- Kim S. J., Kehrl J. H., Burton J., Tendler C. L., Jeang K. T., Danielpour D., Thevenin C., Kim K. Y., Sporn M. B., Roberts A. B. Transactivation of the transforming growth factor beta 1 (TGF-beta 1) gene by human T lymphotropic virus type 1 tax: a potential mechanism for the increased production of TGF-beta 1 in adult T cell leukemia. J Exp Med. 1990 Jul 1;172(1):121–129. doi: 10.1084/jem.172.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laiho M., DeCaprio J. A., Ludlow J. W., Livingston D. M., Massagué J. Growth inhibition by TGF-beta linked to suppression of retinoblastoma protein phosphorylation. Cell. 1990 Jul 13;62(1):175–185. doi: 10.1016/0092-8674(90)90251-9. [DOI] [PubMed] [Google Scholar]
- Li L., Hu J. S., Olson E. N. Different members of the jun proto-oncogene family exhibit distinct patterns of expression in response to type beta transforming growth factor. J Biol Chem. 1990 Jan 25;265(3):1556–1562. [PubMed] [Google Scholar]
- Lyons R. M., Gentry L. E., Purchio A. F., Moses H. L. Mechanism of activation of latent recombinant transforming growth factor beta 1 by plasmin. J Cell Biol. 1990 Apr;110(4):1361–1367. doi: 10.1083/jcb.110.4.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffrey T. A., Falcone D. J., Brayton C. F., Agarwal L. A., Welt F. G., Weksler B. B. Transforming growth factor-beta activity is potentiated by heparin via dissociation of the transforming growth factor-beta/alpha 2-macroglobulin inactive complex. J Cell Biol. 1989 Jul;109(1):441–448. doi: 10.1083/jcb.109.1.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson J. M., Sawamura S. J., Ogawa Y., Dineley K., Carrillo P., Piez K. A. The growth inhibitor of African green monkey (BSC-1) cells is transforming growth factors beta 1 and beta 2. Biochemistry. 1989 Apr 18;28(8):3442–3447. doi: 10.1021/bi00434a044. [DOI] [PubMed] [Google Scholar]
- Miyazono K., Heldin C. H. Role for carbohydrate structures in TGF-beta 1 latency. Nature. 1989 Mar 9;338(6211):158–160. doi: 10.1038/338158a0. [DOI] [PubMed] [Google Scholar]
- Moses H. L., Branum E. L., Proper J. A., Robinson R. A. Transforming growth factor production by chemically transformed cells. Cancer Res. 1981 Jul;41(7):2842–2848. [PubMed] [Google Scholar]
- Newcom S. R., Kadin M. E., Ansari A. A., Diehl V. L-428 nodular sclerosing Hodgkin's cell secretes a unique transforming growth factor-beta active at physiologic pH. J Clin Invest. 1988 Dec;82(6):1915–1921. doi: 10.1172/JCI113810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcom S. R., Kadin M. E., Ansari A. A. Production of transforming growth factor-beta activity by Ki-1 positive lymphoma cells and analysis of its role in the regulation of Ki-1 positive lymphoma growth. Am J Pathol. 1988 Jun;131(3):569–577. [PMC free article] [PubMed] [Google Scholar]
- Newcom S. R., Kadin M. E., Phillips C. L-428 Reed-Sternberg cells and mononuclear Hodgkin's cells arise from a single cloned mononuclear cell. Int J Cell Cloning. 1988 Nov;6(6):417–431. doi: 10.1002/stem.5530060606. [DOI] [PubMed] [Google Scholar]
- Newcom S. R., Kadin M. E., Phillips C. L-428 Reed-Sternberg cells and mononuclear Hodgkin's cells arise from a single cloned mononuclear cell. Int J Cell Cloning. 1988 Nov;6(6):417–431. doi: 10.1002/stem.5530060606. [DOI] [PubMed] [Google Scholar]
- Newcom S. R., Muth L. H., Parker E. T. Production of monoclonal antibodies that detect Hodgkin's high molecular weight transforming growth factor-beta. Blood. 1990 Jun 15;75(12):2434–2437. [PubMed] [Google Scholar]
- Newcom S. R., O'Rourke L. Potentiation of fibroblast growth by nodular sclerosing Hodgkin's disease cell cultures. Blood. 1982 Jul;60(1):228–237. [PubMed] [Google Scholar]
- Newcom S. R. The Hodgkin's cell in nodular sclerosis does not release interleukin-1. J Lab Clin Med. 1985 Feb;105(2):170–177. [PubMed] [Google Scholar]
- Pertovaara L., Sistonen L., Bos T. J., Vogt P. K., Keski-Oja J., Alitalo K. Enhanced jun gene expression is an early genomic response to transforming growth factor beta stimulation. Mol Cell Biol. 1989 Mar;9(3):1255–1262. doi: 10.1128/mcb.9.3.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietenpol J. A., Holt J. T., Stein R. W., Moses H. L. Transforming growth factor beta 1 suppression of c-myc gene transcription: role in inhibition of keratinocyte proliferation. Proc Natl Acad Sci U S A. 1990 May;87(10):3758–3762. doi: 10.1073/pnas.87.10.3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietenpol J. A., Stein R. W., Moran E., Yaciuk P., Schlegel R., Lyons R. M., Pittelkow M. R., Münger K., Howley P. M., Moses H. L. TGF-beta 1 inhibition of c-myc transcription and growth in keratinocytes is abrogated by viral transforming proteins with pRB binding domains. Cell. 1990 Jun 1;61(5):777–785. doi: 10.1016/0092-8674(90)90188-k. [DOI] [PubMed] [Google Scholar]
- Press R. D., Misra A., Gillaspy G., Samols D., Goldthwait D. A. Control of the expression of c-sis mRNA in human glioblastoma cells by phorbol ester and transforming growth factor beta 1. Cancer Res. 1989 Jun 1;49(11):2914–2920. [PubMed] [Google Scholar]
- Roberts A. B., Frolik C. A., Anzano M. A., Sporn M. B. Transforming growth factors from neoplastic and nonneoplastic tissues. Fed Proc. 1983 Jun;42(9):2621–2626. [PubMed] [Google Scholar]
- Schwab U., Stein H., Gerdes J., Lemke H., Kirchner H., Schaadt M., Diehl V. Production of a monoclonal antibody specific for Hodgkin and Sternberg-Reed cells of Hodgkin's disease and a subset of normal lymphoid cells. Nature. 1982 Sep 2;299(5878):65–67. doi: 10.1038/299065a0. [DOI] [PubMed] [Google Scholar]
- Schwarz L. C., Gingras M. C., Goldberg G., Greenberg A. H., Wright J. A. Loss of growth factor dependence and conversion of transforming growth factor-beta 1 inhibition to stimulation in metastatic H-ras-transformed murine fibroblasts. Cancer Res. 1988 Dec 15;48(24 Pt 1):6999–7003. [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Transforming growth factor-beta. Multiple actions and potential clinical applications. JAMA. 1989 Aug 18;262(7):938–941. doi: 10.1001/jama.262.7.938. [DOI] [PubMed] [Google Scholar]
- Stein H., Mason D. Y., Gerdes J., O'Connor N., Wainscoat J., Pallesen G., Gatter K., Falini B., Delsol G., Lemke H. The expression of the Hodgkin's disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood. 1985 Oct;66(4):848–858. [PubMed] [Google Scholar]
- Terzaghi-Howe M. Changes in response to, and production of, transforming growth factor type beta during neoplastic progression in cultured rat tracheal epithelial cells. Carcinogenesis. 1989 Jun;10(6):973–980. doi: 10.1093/carcin/10.6.973. [DOI] [PubMed] [Google Scholar]
- Tucker R. F., Shipley G. D., Moses H. L., Holley R. W. Growth inhibitor from BSC-1 cells closely related to platelet type beta transforming growth factor. Science. 1984 Nov 9;226(4675):705–707. doi: 10.1126/science.6093254. [DOI] [PubMed] [Google Scholar]
- Wakefield L. M., Smith D. M., Flanders K. C., Sporn M. B. Latent transforming growth factor-beta from human platelets. A high molecular weight complex containing precursor sequences. J Biol Chem. 1988 Jun 5;263(16):7646–7654. [PubMed] [Google Scholar]



