Abstract
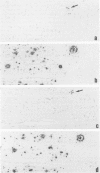
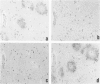
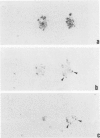
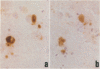
A central unresolved issue in Alzheimer's disease is the origin of the extracellular amyloid beta protein (A beta P) found in senile plaques and its relationship to the dystrophic neurites that intimately surround it. Here the presence and distribution within senile plaques of various epitopes of the beta-amyloid precursor protein (APP) are compared with the distribution of A beta P itself and markers for plaque neurites. Several principal findings emerge: 1) antibodies to regions of APP outside of A beta P ('APP antibodies') recognize only a subgroup of senile plaques; 2) within these plaques, APP antibodies label discrete globular and granular structures morphologically resembling neurites; 3) virtually all of the plaques labeled by APP antibodies also contain neurites reactive with antibodies to tau; 4) double labeling with anti-tau and an APP antibody shows that the neuritelike profiles stained by the APP antibody are always closely associated with tau-positive neurites within the same plaque and that a minority of profiles appear to be labeled by both antibodies; and 5) antibodies to different regions throughout APP label the same profiles within plaques, suggesting the presence of the full-length precursor. The authors conclude that only a subgroup of senile plaques contain APP epitopes and that the immunostained structures are neurites. Because many A beta P-containing plaques in neocortex, cerebellum, and striatum were found to be devoid of any APP labeling, as were vascular A beta P deposits, it is unlikely that the extracellular A beta P is principally derived from the APP found within dystrophic neurites. The immunodetection of apparently full-length APP, an axonally transported protein, in selected plaque neurites provides yet another protein marker of neuritic dystrophy, possibly indicative of an aberrant regenerative response.
Full text
PDF


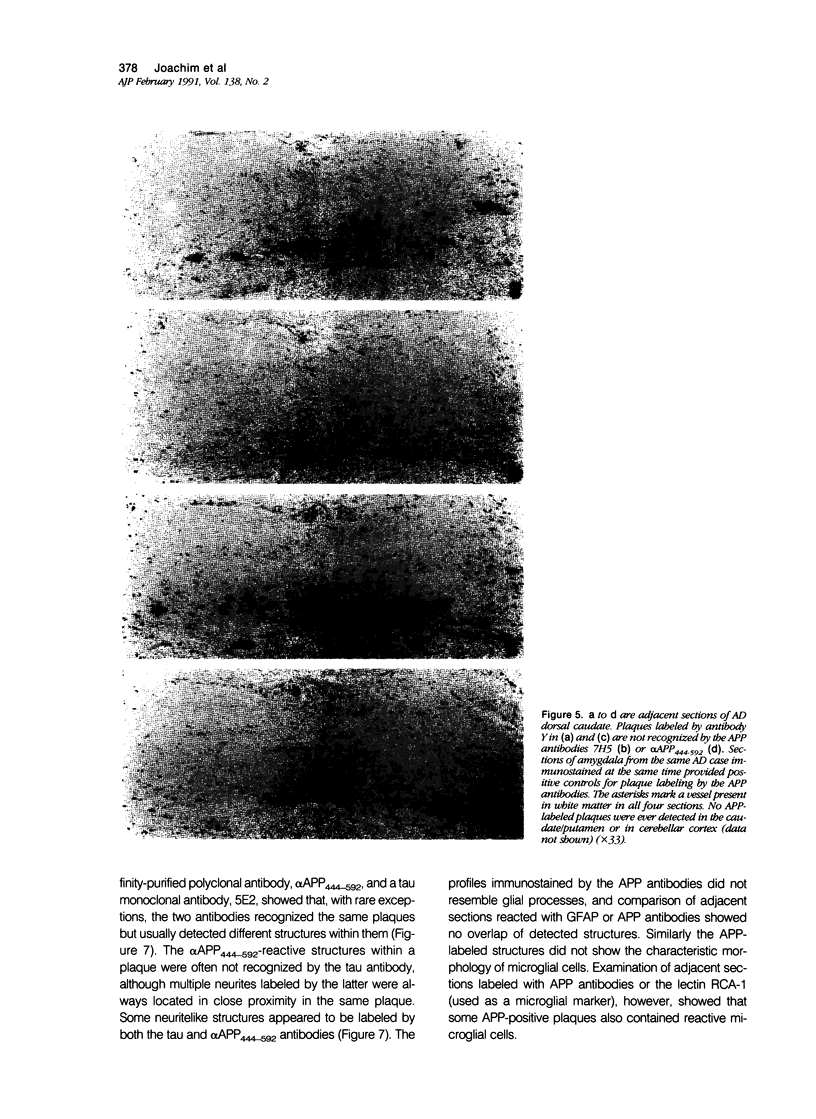
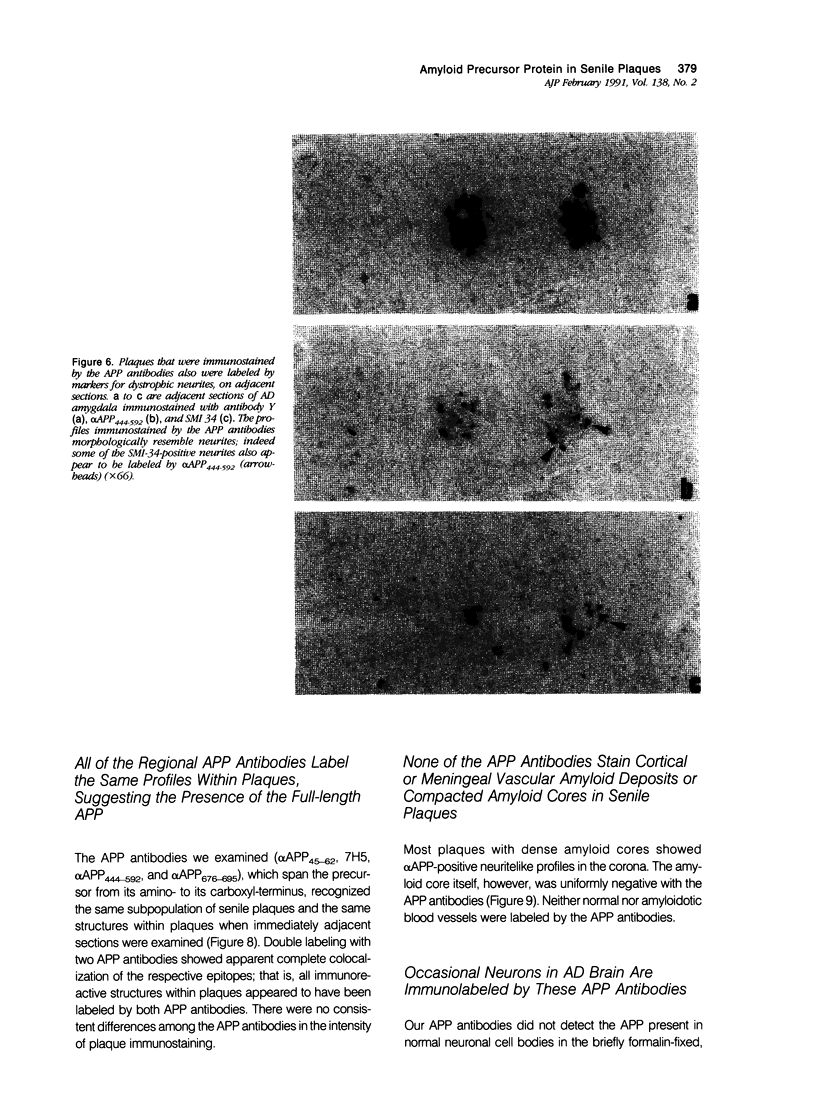
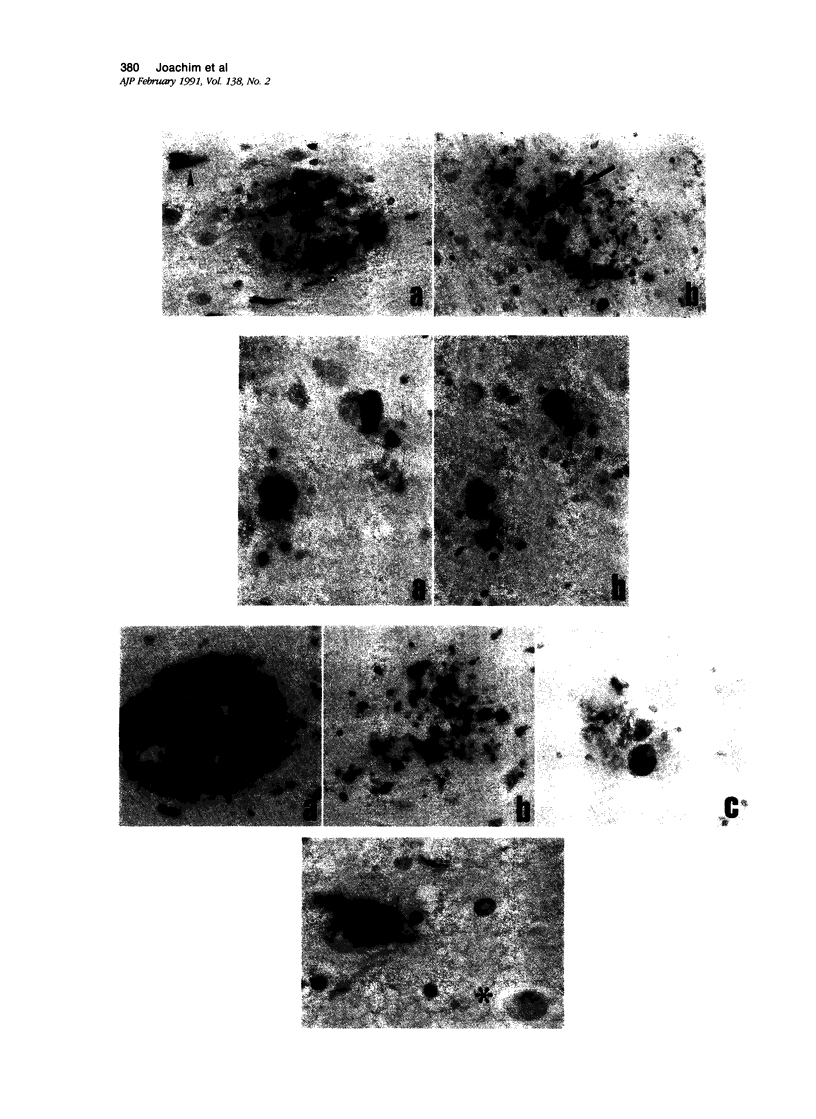




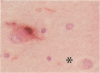
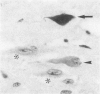
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham C. R., Selkoe D. J., Potter H. Immunochemical identification of the serine protease inhibitor alpha 1-antichymotrypsin in the brain amyloid deposits of Alzheimer's disease. Cell. 1988 Feb 26;52(4):487–501. doi: 10.1016/0092-8674(88)90462-x. [DOI] [PubMed] [Google Scholar]
- Anderton B. H., Breinburg D., Downes M. J., Green P. J., Tomlinson B. E., Ulrich J., Wood J. N., Kahn J. Monoclonal antibodies show that neurofibrillary tangles and neurofilaments share antigenic determinants. Nature. 1982 Jul 1;298(5869):84–86. doi: 10.1038/298084a0. [DOI] [PubMed] [Google Scholar]
- Arai H., Lee V. M., Otvos L., Jr, Greenberg B. D., Lowery D. E., Sharma S. K., Schmidt M. L., Trojanowski J. Q. Defined neurofilament, tau, and beta-amyloid precursor protein epitopes distinguish Alzheimer from non-Alzheimer senile plaques. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2249–2253. doi: 10.1073/pnas.87.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahmanyar S., Higgins G. A., Goldgaber D., Lewis D. A., Morrison J. H., Wilson M. C., Shankar S. K., Gajdusek D. C. Localization of amyloid beta protein messenger RNA in brains from patients with Alzheimer's disease. Science. 1987 Jul 3;237(4810):77–80. doi: 10.1126/science.3299701. [DOI] [PubMed] [Google Scholar]
- Behrouz N., Defossez A., Delacourte A., Mazzuca M. Cortical beta-amyloid. Nature. 1990 Apr 5;344(6266):497–497. doi: 10.1038/344497a0. [DOI] [PubMed] [Google Scholar]
- Benowitz L. I., Rodriguez W., Paskevich P., Mufson E. J., Schenk D., Neve R. L. The amyloid precursor protein is concentrated in neuronal lysosomes in normal and Alzheimer disease subjects. Exp Neurol. 1989 Dec;106(3):237–250. doi: 10.1016/0014-4886(89)90156-8. [DOI] [PubMed] [Google Scholar]
- Bugiani O., Giaccone G., Frangione B., Ghetti B., Tagliavini F. Alzheimer patients: preamyloid deposits are more widely distributed than senile plaques throughout the central nervous system. Neurosci Lett. 1989 Sep 11;103(3):263–268. doi: 10.1016/0304-3940(89)90110-9. [DOI] [PubMed] [Google Scholar]
- Cole G., Masliah E., Huynh T. V., DeTeresa R., Terry R. D., Okuda C., Saitoh T. An antiserum against amyloid beta-protein precursor detects a unique peptide in Alzheimer brain. Neurosci Lett. 1989 May 22;100(1-3):340–346. doi: 10.1016/0304-3940(89)90710-6. [DOI] [PubMed] [Google Scholar]
- Coria F., Castaño E., Prelli F., Larrondo-Lillo M., van Duinen S., Shelanski M. L., Frangione B. Isolation and characterization of amyloid P component from Alzheimer's disease and other types of cerebral amyloidosis. Lab Invest. 1988 Apr;58(4):454–458. [PubMed] [Google Scholar]
- Dickson D. W., Farlo J., Davies P., Crystal H., Fuld P., Yen S. H. Alzheimer's disease. A double-labeling immunohistochemical study of senile plaques. Am J Pathol. 1988 Jul;132(1):86–101. [PMC free article] [PubMed] [Google Scholar]
- Eikelenboom P., Hack C. E., Rozemuller J. M., Stam F. C. Complement activation in amyloid plaques in Alzheimer's dementia. Virchows Arch B Cell Pathol Incl Mol Pathol. 1989;56(4):259–262. doi: 10.1007/BF02890024. [DOI] [PubMed] [Google Scholar]
- Geddes J. W., Anderson K. J., Cotman C. W. Senile plaques as aberrant sprout-stimulating structures. Exp Neurol. 1986 Dec;94(3):767–776. doi: 10.1016/0014-4886(86)90254-2. [DOI] [PubMed] [Google Scholar]
- Ghiso J., Tagliavini F., Timmers W. F., Frangione B. Alzheimer's disease amyloid precursor protein is present in senile plaques and cerebrospinal fluid: immunohistochemical and biochemical characterization. Biochem Biophys Res Commun. 1989 Aug 30;163(1):430–437. doi: 10.1016/0006-291x(89)92154-2. [DOI] [PubMed] [Google Scholar]
- Giaccone G., Tagliavini F., Linoli G., Bouras C., Frigerio L., Frangione B., Bugiani O. Down patients: extracellular preamyloid deposits precede neuritic degeneration and senile plaques. Neurosci Lett. 1989 Feb 13;97(1-2):232–238. doi: 10.1016/0304-3940(89)90169-9. [DOI] [PubMed] [Google Scholar]
- Ihara Y., Abraham C., Selkoe D. J. Antibodies to paired helical filaments in Alzheimer's disease do not recognize normal brain proteins. Nature. 1983 Aug 25;304(5928):727–730. doi: 10.1038/304727a0. [DOI] [PubMed] [Google Scholar]
- Ishii T., Kametani F., Haga S., Sato M. The immunohistochemical demonstration of subsequences of the precursor of the amyloid A4 protein in senile plaques in Alzheimer's disease. Neuropathol Appl Neurobiol. 1989 Mar-Apr;15(2):135–147. doi: 10.1111/j.1365-2990.1989.tb01216.x. [DOI] [PubMed] [Google Scholar]
- Itagaki S., McGeer P. L., Akiyama H., Zhu S., Selkoe D. Relationship of microglia and astrocytes to amyloid deposits of Alzheimer disease. J Neuroimmunol. 1989 Oct;24(3):173–182. doi: 10.1016/0165-5728(89)90115-x. [DOI] [PubMed] [Google Scholar]
- Joachim C. L., Mori H., Selkoe D. J. Amyloid beta-protein deposition in tissues other than brain in Alzheimer's disease. Nature. 1989 Sep 21;341(6239):226–230. doi: 10.1038/341226a0. [DOI] [PubMed] [Google Scholar]
- Joachim C. L., Morris J. H., Selkoe D. J. Diffuse senile plaques occur commonly in the cerebellum in Alzheimer's disease. Am J Pathol. 1989 Aug;135(2):309–319. [PMC free article] [PubMed] [Google Scholar]
- Joachim C. L., Morris J. H., Selkoe D. J., Kosik K. S. Tau epitopes are incorporated into a range of lesions in Alzheimer's disease. J Neuropathol Exp Neurol. 1987 Nov;46(6):611–622. doi: 10.1097/00005072-198711000-00001. [DOI] [PubMed] [Google Scholar]
- Khachaturian Z. S. Diagnosis of Alzheimer's disease. Arch Neurol. 1985 Nov;42(11):1097–1105. doi: 10.1001/archneur.1985.04060100083029. [DOI] [PubMed] [Google Scholar]
- Kitt C. A., Price D. L., Struble R. G., Cork L. C., Wainer B. H., Becher M. W., Mobley W. C. Evidence for cholinergic neurites in senile plaques. Science. 1984 Dec 21;226(4681):1443–1445. doi: 10.1126/science.6505701. [DOI] [PubMed] [Google Scholar]
- Koo E. H., Sisodia S. S., Archer D. R., Martin L. J., Weidemann A., Beyreuther K., Fischer P., Masters C. L., Price D. L. Precursor of amyloid protein in Alzheimer disease undergoes fast anterograde axonal transport. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1561–1565. doi: 10.1073/pnas.87.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenders M. B., Peers M. C., Tramu G., Delacourte A., Defossez A., Petit H., Mazzuca M. Dystrophic peptidergic neurites in senile plaques of Alzheimer's disease hippocampus precede formation of paired helical filaments. Brain Res. 1989 Mar 6;481(2):344–349. doi: 10.1016/0006-8993(89)90812-3. [DOI] [PubMed] [Google Scholar]
- Li J. J., McAdam K. P. Human amyloid P component: an elastase inhibitor. Scand J Immunol. 1984 Sep;20(3):219–226. doi: 10.1111/j.1365-3083.1984.tb00995.x. [DOI] [PubMed] [Google Scholar]
- Masters C. L., Beyreuther K. Neuronal origin of cerebral amyloidogenic proteins: their role in Alzheimer's disease and unconventional virus diseases of the nervous system. Ciba Found Symp. 1987;126:49–64. doi: 10.1002/9780470513422.ch4. [DOI] [PubMed] [Google Scholar]
- Masters C. L., Multhaup G., Simms G., Pottgiesser J., Martins R. N., Beyreuther K. Neuronal origin of a cerebral amyloid: neurofibrillary tangles of Alzheimer's disease contain the same protein as the amyloid of plaque cores and blood vessels. EMBO J. 1985 Nov;4(11):2757–2763. doi: 10.1002/j.1460-2075.1985.tb04000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori H., Kondo J., Ihara Y. Ubiquitin is a component of paired helical filaments in Alzheimer's disease. Science. 1987 Mar 27;235(4796):1641–1644. doi: 10.1126/science.3029875. [DOI] [PubMed] [Google Scholar]
- Morrison J. H., Rogers J., Scherr S., Benoit R., Bloom F. E. Somatostatin immunoreactivity in neuritic plaques of Alzheimer's patients. Nature. 1985 Mar 7;314(6006):90–92. doi: 10.1038/314090a0. [DOI] [PubMed] [Google Scholar]
- Motte J., Williams R. S. Age-related changes in the density and morphology of plaques and neurofibrillary tangles in Down syndrome brain. Acta Neuropathol. 1989;77(5):535–546. doi: 10.1007/BF00687256. [DOI] [PubMed] [Google Scholar]
- Oltersdorf T., Fritz L. C., Schenk D. B., Lieberburg I., Johnson-Wood K. L., Beattie E. C., Ward P. J., Blacher R. W., Dovey H. F., Sinha S. The secreted form of the Alzheimer's amyloid precursor protein with the Kunitz domain is protease nexin-II. Nature. 1989 Sep 14;341(6238):144–147. doi: 10.1038/341144a0. [DOI] [PubMed] [Google Scholar]
- Oltersdorf T., Ward P. J., Henriksson T., Beattie E. C., Neve R., Lieberburg I., Fritz L. C. The Alzheimer amyloid precursor protein. Identification of a stable intermediate in the biosynthetic/degradative pathway. J Biol Chem. 1990 Mar 15;265(8):4492–4497. [PubMed] [Google Scholar]
- Palmert M. R., Podlisny M. B., Witker D. S., Oltersdorf T., Younkin L. H., Selkoe D. J., Younkin S. G. Antisera to an amino-terminal peptide detect the amyloid protein precursor of Alzheimer's disease and recognize senile plaques. Biochem Biophys Res Commun. 1988 Oct 14;156(1):432–437. doi: 10.1016/s0006-291x(88)80859-3. [DOI] [PubMed] [Google Scholar]
- Palmert M. R., Podlisny M. B., Witker D. S., Oltersdorf T., Younkin L. H., Selkoe D. J., Younkin S. G. The beta-amyloid protein precursor of Alzheimer disease has soluble derivatives found in human brain and cerebrospinal fluid. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6338–6342. doi: 10.1073/pnas.86.16.6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry G., Lipphardt S., Mulvihill P., Kancherla M., Mijares M., Gambetti P., Sharma S., Maggiora L., Cornette J., Lobl T. Amyloid precursor protein in senile plaques of Alzheimer disease. Lancet. 1988 Sep 24;2(8613):746–746. doi: 10.1016/s0140-6736(88)90219-x. [DOI] [PubMed] [Google Scholar]
- Powers R. E., Struble R. G., Casanova M. F., O'Connor D. T., Kitt C. A., Price D. L. Innervation of human hippocampus by noradrenergic systems: normal anatomy and structural abnormalities in aging and in Alzheimer's disease. Neuroscience. 1988 May;25(2):401–417. doi: 10.1016/0306-4522(88)90248-5. [DOI] [PubMed] [Google Scholar]
- Schmechel D. E., Goldgaber D., Burkhart D. S., Gilbert J. R., Gajdusek D. C., Roses A. D. Cellular localization of messenger RNA encoding amyloid-beta-protein in normal tissue and in Alzheimer disease. Alzheimer Dis Assoc Disord. 1988;2(2):96–111. doi: 10.1097/00002093-198802020-00002. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J., Abraham C. R., Podlisny M. B., Duffy L. K. Isolation of low-molecular-weight proteins from amyloid plaque fibers in Alzheimer's disease. J Neurochem. 1986 Jun;46(6):1820–1834. doi: 10.1111/j.1471-4159.1986.tb08501.x. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J., Ihara Y., Salazar F. J. Alzheimer's disease: insolubility of partially purified paired helical filaments in sodium dodecyl sulfate and urea. Science. 1982 Mar 5;215(4537):1243–1245. doi: 10.1126/science.6120571. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J. Molecular pathology of amyloidogenic proteins and the role of vascular amyloidosis in Alzheimer's disease. Neurobiol Aging. 1989 Sep-Oct;10(5):387–395. doi: 10.1016/0197-4580(89)90072-9. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J., Podlisny M. B., Joachim C. L., Vickers E. A., Lee G., Fritz L. C., Oltersdorf T. Beta-amyloid precursor protein of Alzheimer disease occurs as 110- to 135-kilodalton membrane-associated proteins in neural and nonneural tissues. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7341–7345. doi: 10.1073/pnas.85.19.7341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji M., Hirai S., Yamaguchi H., Harigaya Y., Kawarabayashi T. Amyloid beta-protein precursor accumulates in dystrophic neurites of senile plaques in Alzheimer-type dementia. Brain Res. 1990 Mar 26;512(1):164–168. doi: 10.1016/0006-8993(90)91187-l. [DOI] [PubMed] [Google Scholar]
- Siman R., Card J. P., Nelson R. B., Davis L. G. Expression of beta-amyloid precursor protein in reactive astrocytes following neuronal damage. Neuron. 1989 Sep;3(3):275–285. doi: 10.1016/0896-6273(89)90252-3. [DOI] [PubMed] [Google Scholar]
- Struble R. G., Powers R. E., Casanova M. F., Kitt C. A., Brown E. C., Price D. L. Neuropeptidergic systems in plaques of Alzheimer's disease. J Neuropathol Exp Neurol. 1987 Sep;46(5):567–584. doi: 10.1097/00005072-198709000-00006. [DOI] [PubMed] [Google Scholar]
- Suenaga T., Hirano A., Llena J. F., Ksiezak-Reding H., Yen S. H., Dickson D. W. Modified Bielschowsky and immunocytochemical studies on cerebellar plaques in Alzheimer's disease. J Neuropathol Exp Neurol. 1990 Jan;49(1):31–40. doi: 10.1097/00005072-199001000-00004. [DOI] [PubMed] [Google Scholar]
- Tagliavini F., Giaccone G., Frangione B., Bugiani O. Preamyloid deposits in the cerebral cortex of patients with Alzheimer's disease and nondemented individuals. Neurosci Lett. 1988 Nov 11;93(2-3):191–196. doi: 10.1016/0304-3940(88)90080-8. [DOI] [PubMed] [Google Scholar]
- Tanzi R. E., Gusella J. F., Watkins P. C., Bruns G. A., St George-Hyslop P., Van Keuren M. L., Patterson D., Pagan S., Kurnit D. M., Neve R. L. Amyloid beta protein gene: cDNA, mRNA distribution, and genetic linkage near the Alzheimer locus. Science. 1987 Feb 20;235(4791):880–884. doi: 10.1126/science.2949367. [DOI] [PubMed] [Google Scholar]
- Tate-Ostroff B., Majocha R. E., Marotta C. A. Identification of cellular and extracellular sites of amyloid precursor protein extracytoplasmic domain in normal and Alzheimer disease brains. Proc Natl Acad Sci U S A. 1989 Jan;86(2):745–749. doi: 10.1073/pnas.86.2.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Nostrand W. E., Cunningham D. D. Purification of protease nexin II from human fibroblasts. J Biol Chem. 1987 Jun 25;262(18):8508–8514. [PubMed] [Google Scholar]
- Van Nostrand W. E., Wagner S. L., Suzuki M., Choi B. H., Farrow J. S., Geddes J. W., Cotman C. W., Cunningham D. D. Protease nexin-II, a potent antichymotrypsin, shows identity to amyloid beta-protein precursor. Nature. 1989 Oct 12;341(6242):546–549. doi: 10.1038/341546a0. [DOI] [PubMed] [Google Scholar]
- Weidemann A., König G., Bunke D., Fischer P., Salbaum J. M., Masters C. L., Beyreuther K. Identification, biogenesis, and localization of precursors of Alzheimer's disease A4 amyloid protein. Cell. 1989 Apr 7;57(1):115–126. doi: 10.1016/0092-8674(89)90177-3. [DOI] [PubMed] [Google Scholar]
- Wisniewski H. M., Bancher C., Barcikowska M., Wen G. Y., Currie J. Spectrum of morphological appearance of amyloid deposits in Alzheimer's disease. Acta Neuropathol. 1989;78(4):337–347. doi: 10.1007/BF00688170. [DOI] [PubMed] [Google Scholar]
- Wisniewski H. M., Wegiel J., Wang K. C., Kujawa M., Lach B. Ultrastructural studies of the cells forming amyloid fibers in classical plaques. Can J Neurol Sci. 1989 Nov;16(4 Suppl):535–542. doi: 10.1017/s0317167100029887. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H., Hirai S., Morimatsu M., Shoji M., Harigaya Y. Diffuse type of senile plaques in the brains of Alzheimer-type dementia. Acta Neuropathol. 1988;77(2):113–119. doi: 10.1007/BF00687420. [DOI] [PubMed] [Google Scholar]