Abstract
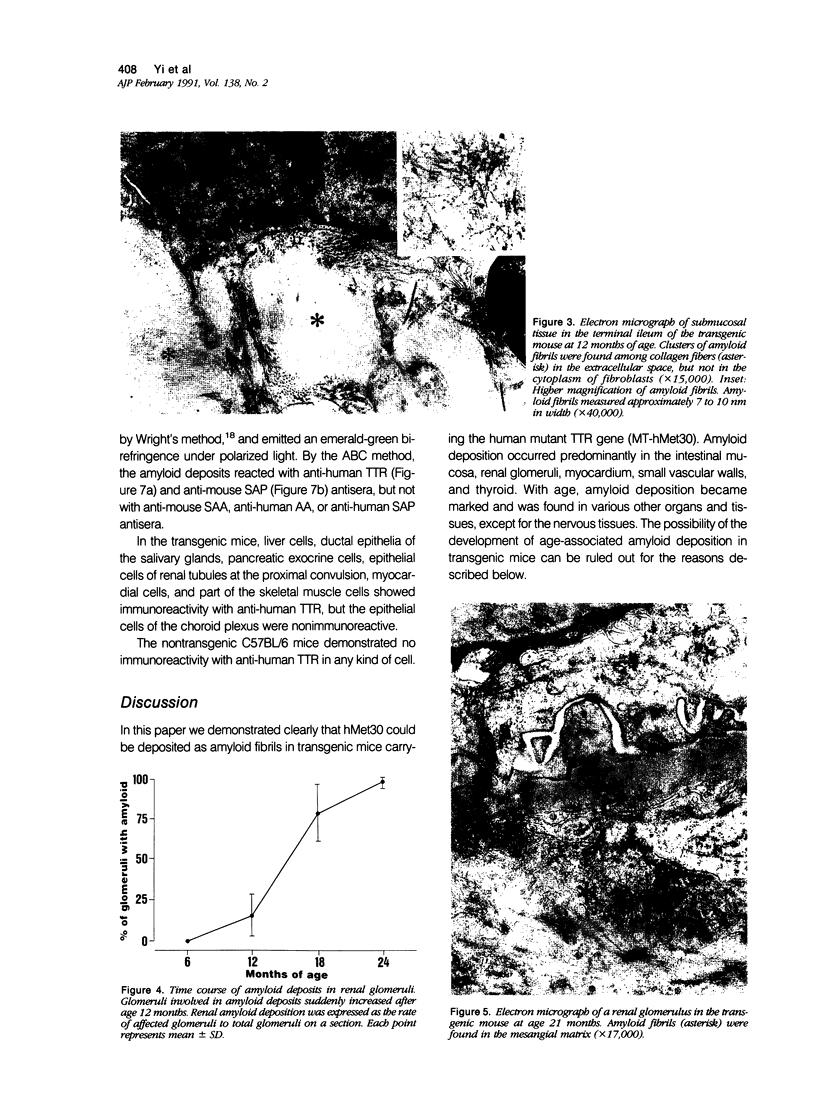
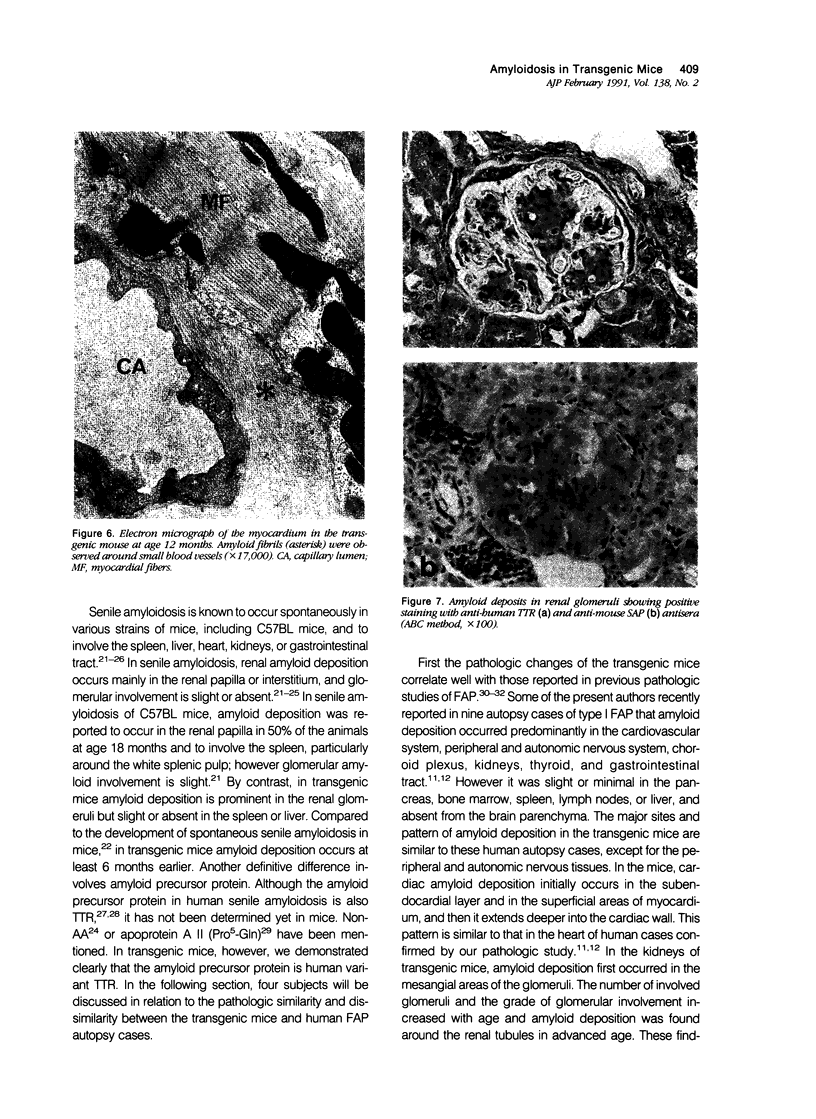
To analyze the pathologic processes of amyloid deposition in type I familial amyloidotic polyneuropathy (FAP), mice were made transgenic by introducing the human mutant transthyretin (TTR) gene. In these transgenic mice, amyloid deposition started in the gastrointestinal tract, cardiovascular system, and kidneys 6 months after birth and extended to various other organs and tissues with advancing age. At age 24 months, the pattern of amyloid deposition was similar to that observed in human autopsy cases of FAP, except for its absence in the choroid plexus and in the peripheral and autonomic nervous systems. Amyloid deposition was shown to be composed of human mutant TTR and, in addition, mouse serum amyloid P component. These results clearly indicate that human variant TTR produced in transgenic mice deposits is a major component of amyloid fibrils in various organs and tissues. Thus this animal model is useful for analyzing how amyloid deposition initiates and proceeds in FAP.
Full text
PDF


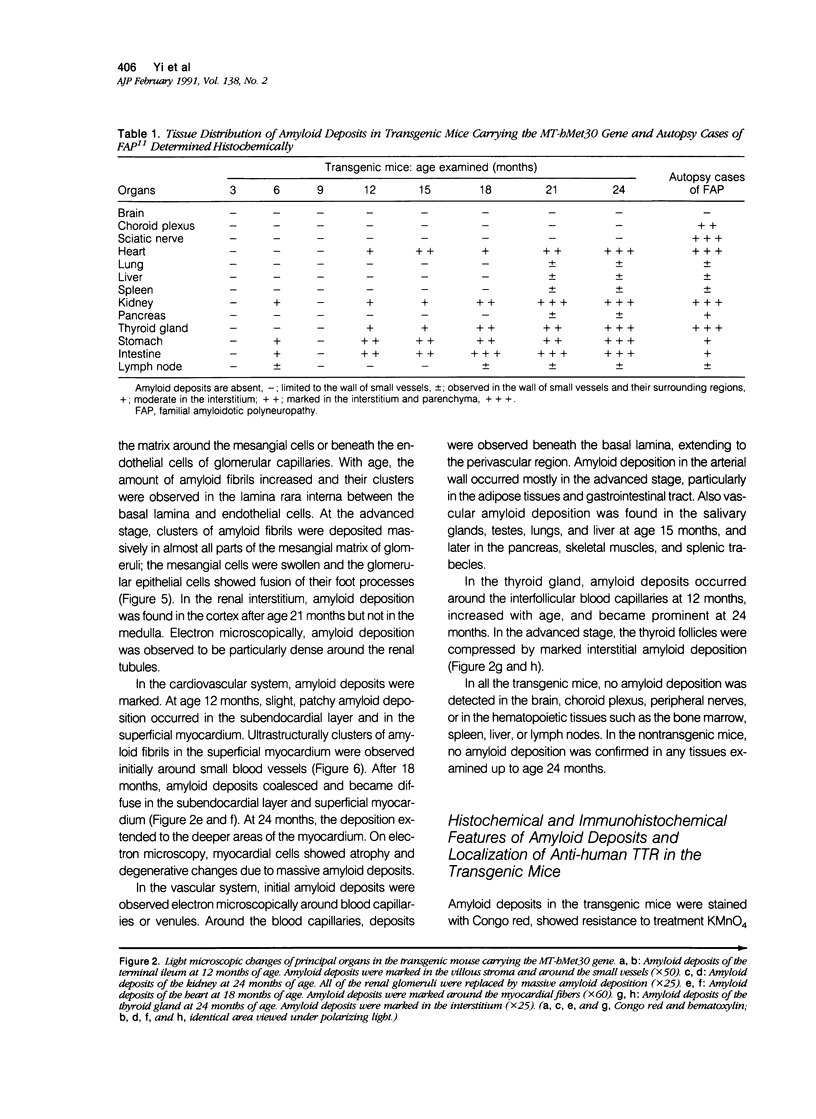
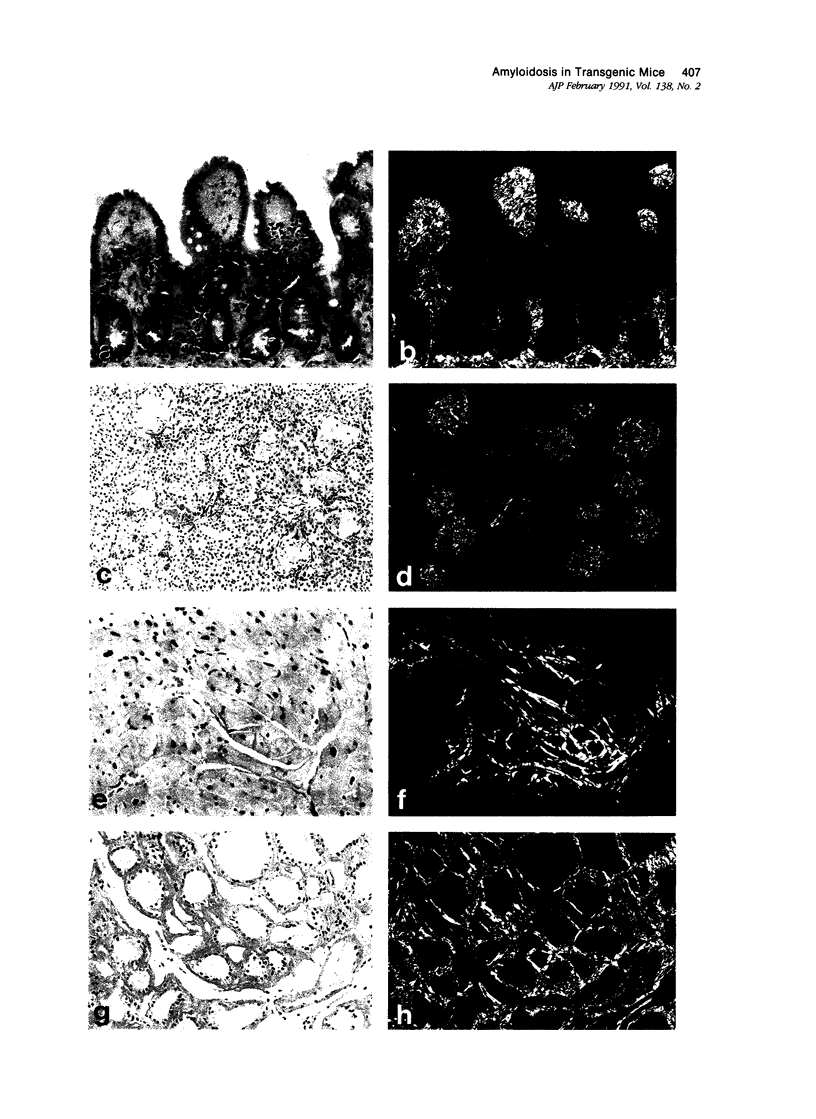



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDRADE C. A peculiar form of peripheral neuropathy; familiar atypical generalized amyloidosis with special involvement of the peripheral nerves. Brain. 1952 Sep;75(3):408–427. doi: 10.1093/brain/75.3.408. [DOI] [PubMed] [Google Scholar]
- Araki S., Mawatari S., Ohta M., Nakajima A., Kuroiwa Y. Polyneuritic amyloidosis in a Japanese family. Arch Neurol. 1968 Jun;18(6):593–602. doi: 10.1001/archneur.1968.00470360015001. [DOI] [PubMed] [Google Scholar]
- Araki S. Type I familial amyloidotic polyneuropathy (Japanese type). Brain Dev. 1984;6(2):128–133. doi: 10.1016/s0387-7604(84)80061-3. [DOI] [PubMed] [Google Scholar]
- Benson M. D. Familial amyloidotic polyneuropathy. Trends Neurosci. 1989 Mar;12(3):88–92. doi: 10.1016/0166-2236(89)90162-8. [DOI] [PubMed] [Google Scholar]
- Chai C. K. Spontaneous amyloidosis in LLC mice. Am J Pathol. 1978 Feb;90(2):381–398. [PMC free article] [PubMed] [Google Scholar]
- Cornwell G. G., 3rd, Sletten K., Johansson B., Westermark P. Evidence that the amyloid fibril protein in senile systemic amyloidosis is derived from normal prealbumin. Biochem Biophys Res Commun. 1988 Jul 29;154(2):648–653. doi: 10.1016/0006-291x(88)90188-x. [DOI] [PubMed] [Google Scholar]
- Costa P. P., Figueira A. S., Bravo F. R. Amyloid fibril protein related to prealbumin in familial amyloidotic polyneuropathy. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4499–4503. doi: 10.1073/pnas.75.9.4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwulet F. E., Benson M. D. Primary structure of an amyloid prealbumin and its plasma precursor in a heredofamilial polyneuropathy of Swedish origin. Proc Natl Acad Sci U S A. 1984 Feb;81(3):694–698. doi: 10.1073/pnas.81.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenner G. G., Eanes E. D., Bladen H. A., Linke R. P., Termine J. D. Beta-pleated sheet fibrils. A comparison of native amyloid with synthetic protein fibrils. J Histochem Cytochem. 1974 Dec;22(12):1141–1158. doi: 10.1177/22.12.1141. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Murphy M. A. Amyloidosis of the nervous system. J Neurol Sci. 1989 Dec;94(1-3):1–28. doi: 10.1016/0022-510x(89)90214-1. [DOI] [PubMed] [Google Scholar]
- HORTAJDA S., FILIPE I., DURANTE S. PORTUGUESE POLYNEURITIC FAMILIAL TYPE OF AMYLOIDOSIS. Pathol Microbiol (Basel) 1964;27:809–825. [PubMed] [Google Scholar]
- Herbert J., Wilcox J. N., Pham K. T., Fremeau R. T., Jr, Zeviani M., Dwork A., Soprano D. R., Makover A., Goodman D. S., Zimmerman E. A. Transthyretin: a choroid plexus-specific transport protein in human brain. The 1986 S. Weir Mitchell award. Neurology. 1986 Jul;36(7):900–911. doi: 10.1212/wnl.36.7.900. [DOI] [PubMed] [Google Scholar]
- Higuchi K., Matsumura A., Honma A., Takeshita S., Hashimoto K., Hosokawa M., Yasuhira K., Takeda T. Systemic senile amyloid in senescence-accelerated mice. A unique fibril protein demonstrated in tissues from various organs by the unlabeled immunoperoxidase method. Lab Invest. 1983 Feb;48(2):231–240. [PubMed] [Google Scholar]
- Hooper M., Hardy K., Handyside A., Hunter S., Monk M. HPRT-deficient (Lesch-Nyhan) mouse embryos derived from germline colonization by cultured cells. Nature. 1987 Mar 19;326(6110):292–295. doi: 10.1038/326292a0. [DOI] [PubMed] [Google Scholar]
- Ikeda S., Hanyu N., Hongo M., Yoshioka J., Oguchi H., Yanagisawa N., Kobayashi T., Tsukagoshi H., Ito N., Yokota T. Hereditary generalized amyloidosis with polyneuropathy. Clinicopathological study of 65 Japanese patients. Brain. 1987 Apr;110(Pt 2):315–337. doi: 10.1093/brain/110.2.315. [DOI] [PubMed] [Google Scholar]
- Kito S., Itoga E., Kamiya K., Kishida T., Yamamura Y. Studies on familial amyloid polyneuropathy in Ogawa Village, Japan. Eur Neurol. 1980;19(3):141–151. doi: 10.1159/000115139. [DOI] [PubMed] [Google Scholar]
- Kuehn M. R., Bradley A., Robertson E. J., Evans M. J. A potential animal model for Lesch-Nyhan syndrome through introduction of HPRT mutations into mice. Nature. 1987 Mar 19;326(6110):295–298. doi: 10.1038/326295a0. [DOI] [PubMed] [Google Scholar]
- Mita S., Maeda S., Ide M., Tsuzuki T., Shimada K., Araki S. Familial amyloidotic polyneuropathy diagnosed by cloned human prealbumin cDNA. Neurology. 1986 Feb;36(2):298–301. doi: 10.1212/wnl.36.2.298. [DOI] [PubMed] [Google Scholar]
- Mita S., Maeda S., Shimada K., Araki S. Cloning and sequence analysis of cDNA for human prealbumin. Biochem Biophys Res Commun. 1984 Oct 30;124(2):558–564. doi: 10.1016/0006-291x(84)91590-0. [DOI] [PubMed] [Google Scholar]
- Pepys M. B., Baltz M. L., de Beer F. C., Dyck R. F., Holford S., Breathnach S. M., Black M. M., Tribe C. R., Evans D. J., Feinstein A. Biology of serum amyloid P component. Ann N Y Acad Sci. 1982;389:286–298. doi: 10.1111/j.1749-6632.1982.tb22144.x. [DOI] [PubMed] [Google Scholar]
- Pepys M. B., Dyck R. F., de Beer F. C., Skinner M., Cohen A. S. Binding of serum amyloid P-component (SAP) by amyloid fibrils. Clin Exp Immunol. 1979 Nov;38(2):284–293. [PMC free article] [PubMed] [Google Scholar]
- Saraiva M. J., Birken S., Costa P. P., Goodman D. S. Amyloid fibril protein in familial amyloidotic polyneuropathy, Portuguese type. Definition of molecular abnormality in transthyretin (prealbumin). J Clin Invest. 1984 Jul;74(1):104–119. doi: 10.1172/JCI111390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki H., Sakaki Y., Matsuo H., Goto I., Kuroiwa Y., Sahashi I., Takahashi A., Shinoda T., Isobe T., Takagi Y. Diagnosis of familial amyloidotic polyneuropathy by recombinant DNA techniques. Biochem Biophys Res Commun. 1984 Dec 14;125(2):636–642. doi: 10.1016/0006-291x(84)90586-2. [DOI] [PubMed] [Google Scholar]
- Scheinberg M. A., Cathcart E. S., Eastcott J. W., Skinner M., Benson M., Shirahama T. The SJL/J mouse: a new model for spontaneous age-associated amyloidosis. I. Morphologic and immunochemical aspects. Lab Invest. 1976 Jul;35(1):47–54. [PubMed] [Google Scholar]
- Shimada K., Maeda S., Murakami T., Nishiguchi S., Tashiro F., Yi S., Wakasugi S., Takahashi K., Yamamura K. Transgenic mouse model of familial amyloidotic polyneuropathy. Mol Biol Med. 1989 Aug;6(4):333–343. [PubMed] [Google Scholar]
- Skinner M., Cathcart E. S., Cohen A. S., Benson M. D. Isolation and identification by sequence analysis of experimentally induced guinea pig amyloid fibrils. J Exp Med. 1974 Sep 1;140(3):871–876. doi: 10.1084/jem.140.3.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner M., Cohen A. S., Shirahama T., Cathcart E. S. P-component (pentagonal unit) of amyloid: isolation, characterization, and sequence analysis. J Lab Clin Med. 1974 Oct;84(4):604–614. [PubMed] [Google Scholar]
- Sletten K., Westermark P., Natvig J. B. Senile cardiac amyloid is related to prealbumin. Scand J Immunol. 1980;12(6):503–506. doi: 10.1111/j.1365-3083.1980.tb00098.x. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- THUNG P. J. Senile amyloidosis in mice. Gerontologia. 1957;1(5):259–279. doi: 10.1159/000210704. [DOI] [PubMed] [Google Scholar]
- Tawara S., Nakazato M., Kangawa K., Matsuo H., Araki S. Identification of amyloid prealbumin variant in familial amyloidotic polyneuropathy (Japanese type). Biochem Biophys Res Commun. 1983 Nov 15;116(3):880–888. doi: 10.1016/s0006-291x(83)80224-1. [DOI] [PubMed] [Google Scholar]
- West W. T., Murphy E. D. Sequence of deposition of amyloid in strain A mice and relationship to renal disease. J Natl Cancer Inst. 1965 Jul;35(1):167–174. doi: 10.1093/jnci/35.1.167. [DOI] [PubMed] [Google Scholar]
- Wright J. R., Calkins E., Humphrey R. L. Potassium permanganate reaction in amyloidosis. A histologic method to assist in differentiating forms of this disease. Lab Invest. 1977 Mar;36(3):274–281. [PubMed] [Google Scholar]
- Yamamura K., Kikutani H., Takahashi N., Taga T., Akira S., Kawai K., Fukuchi K., Kumahara Y., Honjo T., Kishimoto T. Introduction of human gamma 1 immunoglobulin genes into fertilized mouse eggs. J Biochem. 1984 Aug;96(2):357–363. doi: 10.1093/oxfordjournals.jbchem.a134845. [DOI] [PubMed] [Google Scholar]
- Yonezu T., Tsunasawa S., Higuchi K., Kogishi K., Naiki H., Hanada K., Sakiyama F., Takeda T. A molecular-pathologic approach to murine senile amyloidosis. Serum precursor-apolipoprotein A-II variant (Pro5----Gln) presents only in the senile amyloidosis-prone SAM-P/1 and SAM-P/2 mice. Lab Invest. 1987 Jul;57(1):65–70. [PubMed] [Google Scholar]