Abstract
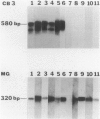
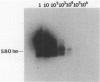
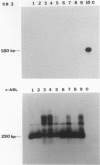
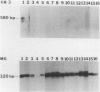
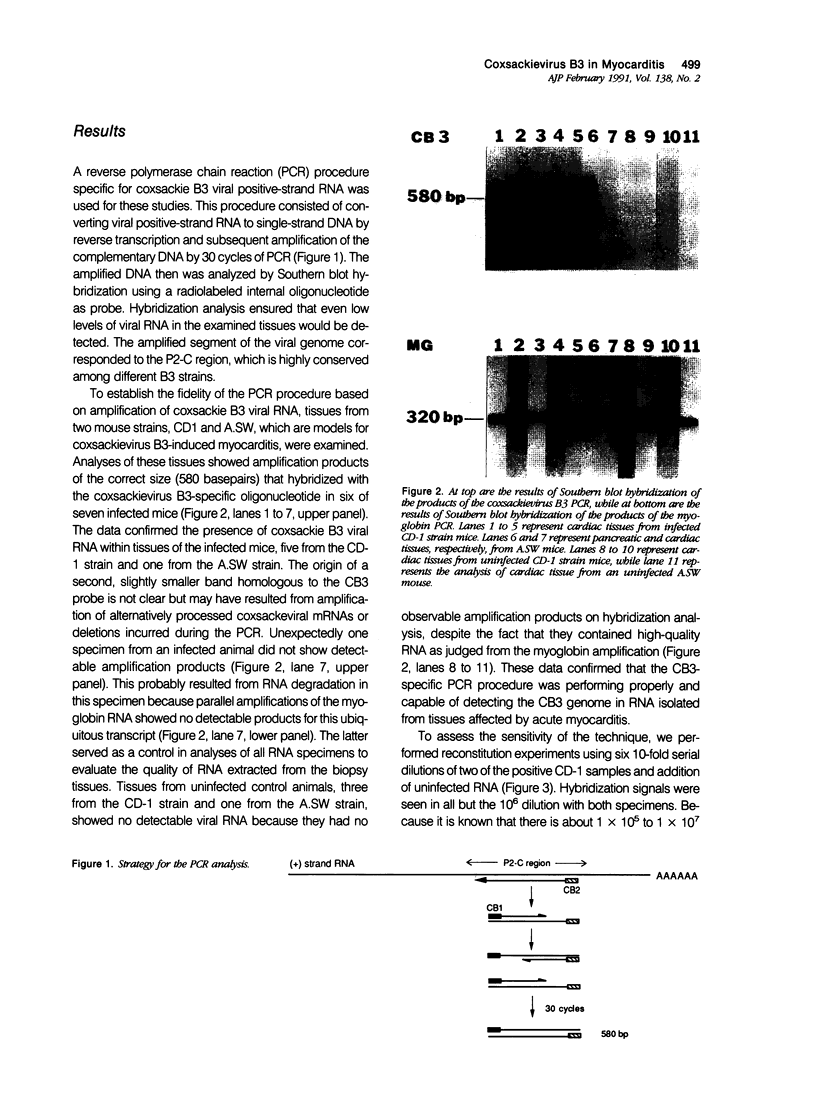
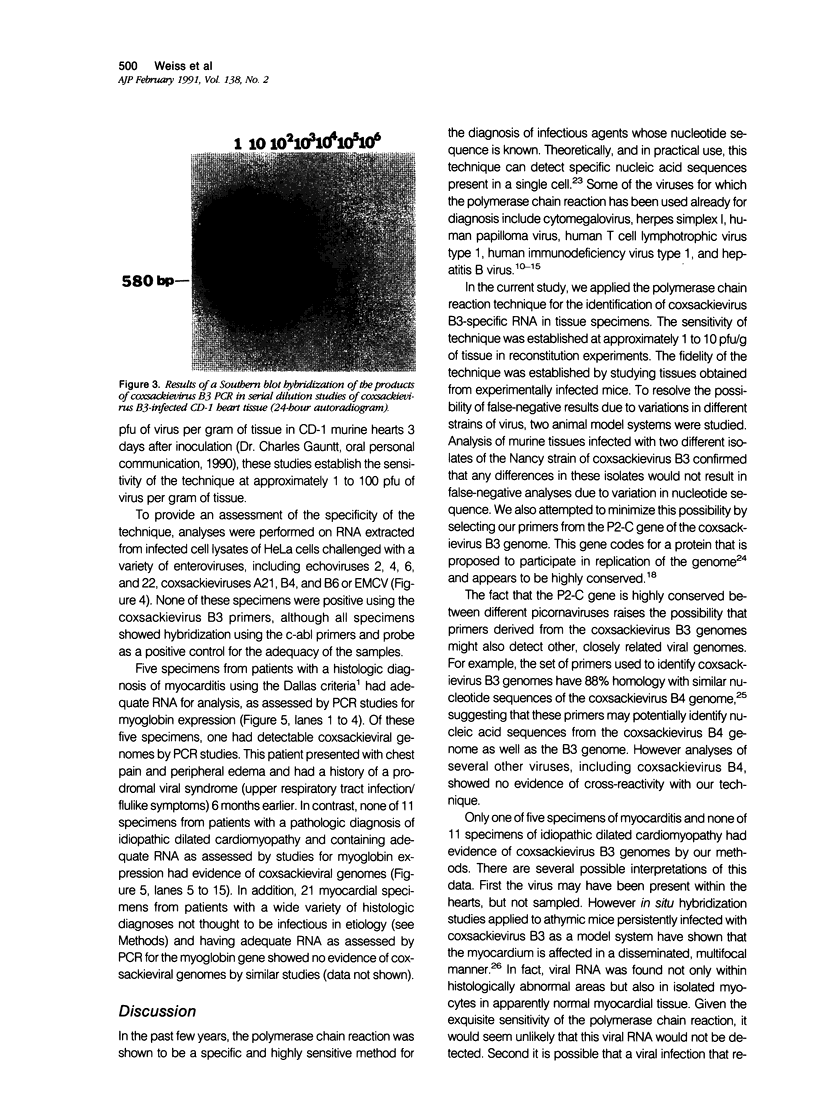
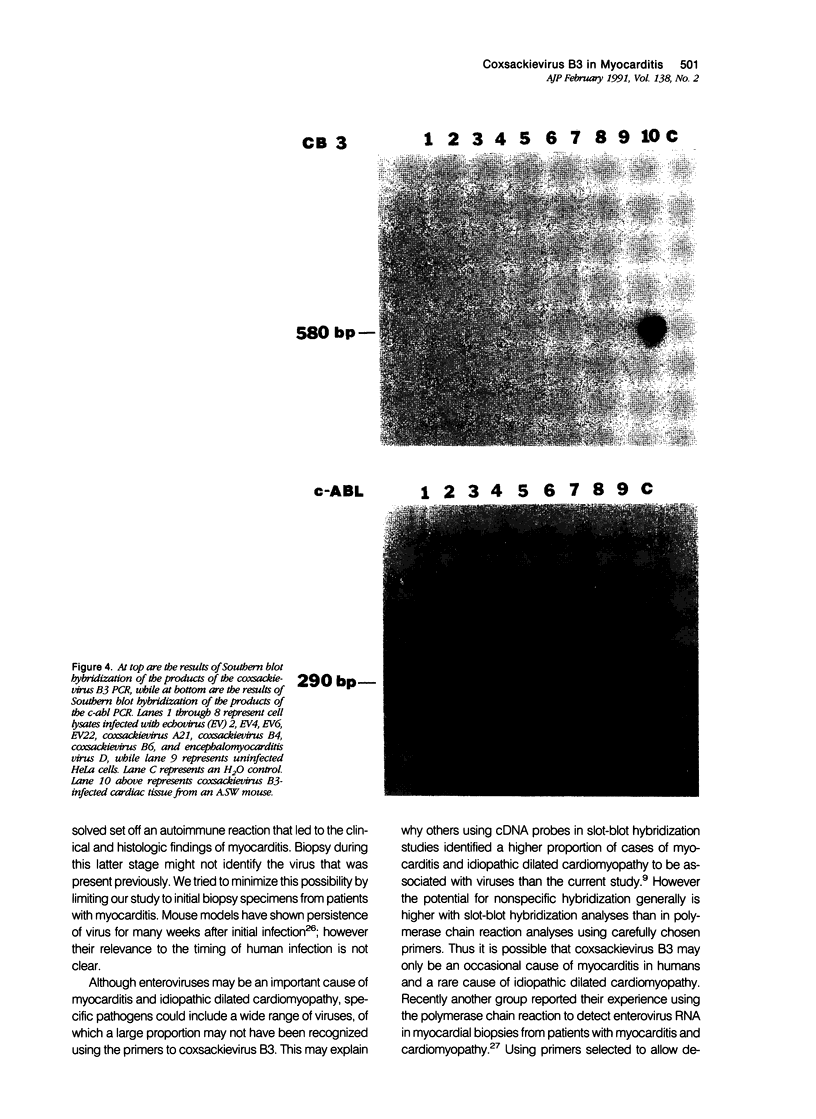
Coxsackievirus B3 is a possible etiologic agent in some forms of myocarditis and idiopathic dilated cardiomyopathy. A method for the detection of coxsackievirus B3 RNA was developed using the polymerase chain reaction based on the amplification of a cDNA copy of the positive-strand viral RNA. The fidelity of the method was established in two murine models for coxsackie B3 myocarditis. All cardiac specimens with adequate RNA for study from coxsackie B3-infected mice contained detectable viral RNA, in contrast to none of control specimens from noninfected mice. The sensitivity of the technique was established at approximately 1 to 100 plaque-forming units of virus per gram of tissue, and the specificity was established as limited to the coxsackievirus B3 serotype among nine viruses tested. In patients with myocarditis, one of five specimens contained detectable viral RNA, whereas none of 11 specimens from patients with idiopathic dilated cardiomyopathy or 21 myocardial specimens from patients with a wide variety of other cardiac disorders contained detectable coxsackie B3 viral RNA. The results show that the polymerase chain reaction is a useful means for detecting coxsackie viral RNA and its application should help in the evaluation of hypotheses concerning the infectious etiology of human myocarditis and idiopathic dilated cardiomyopathy.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abelmann W. H. Viral myocarditis and its sequelae. Annu Rev Med. 1973;24:145–152. doi: 10.1146/annurev.me.24.020173.001045. [DOI] [PubMed] [Google Scholar]
- Aretz H. T., Billingham M. E., Edwards W. D., Factor S. M., Fallon J. T., Fenoglio J. J., Jr, Olsen E. G., Schoen F. J. Myocarditis. A histopathologic definition and classification. Am J Cardiovasc Pathol. 1987 Jan;1(1):3–14. [PubMed] [Google Scholar]
- Bowles N. E., Richardson P. J., Olsen E. G., Archard L. C. Detection of Coxsackie-B-virus-specific RNA sequences in myocardial biopsy samples from patients with myocarditis and dilated cardiomyopathy. Lancet. 1986 May 17;1(8490):1120–1123. doi: 10.1016/s0140-6736(86)91837-4. [DOI] [PubMed] [Google Scholar]
- Cambridge G., MacArthur C. G., Waterson A. P., Goodwin J. F., Oakley C. M. Antibodies to Coxsackie B viruses in congestive cardiomyopathy. Br Heart J. 1979 Jun;41(6):692–696. doi: 10.1136/hrt.41.6.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao M., Xiao X., Egbert B., Darragh T. M., Yen T. S. Rapid detection of cutaneous herpes simplex virus infection with the polymerase chain reaction. J Invest Dermatol. 1989 Mar;92(3):391–392. doi: 10.1111/1523-1747.ep12277232. [DOI] [PubMed] [Google Scholar]
- Chehab F. F., Xiao X., Kan Y. W., Yen T. S. Detection of cytomegalovirus infection in paraffin-embedded tissue specimens with the polymerase chain reaction. Mod Pathol. 1989 Mar;2(2):75–78. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Duggan D. B., Ehrlich G. D., Davey F. P., Kwok S., Sninsky J., Goldberg J., Baltrucki L., Poiesz B. J. HTLV-I-induced lymphoma mimicking Hodgkin's disease. Diagnosis by polymerase chain reaction amplification of specific HTLV-I sequences in tumor DNA. Blood. 1988 Apr;71(4):1027–1032. [PubMed] [Google Scholar]
- Gauntt C. J., Gomez P. T., Duffey P. S., Grant J. A., Trent D. W., Witherspoon S. M., Paque R. E. Characterization and myocarditic capabilities of coxsackievirus B3 variants in selected mouse strains. J Virol. 1984 Nov;52(2):598–605. doi: 10.1128/jvi.52.2.598-605.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskowitz A., Wolfgram L. J., Rose N. R., Beisel K. W. Coxsackievirus B3 murine myocarditis: a pathologic spectrum of myocarditis in genetically defined inbred strains. J Am Coll Cardiol. 1987 Jun;9(6):1311–1319. doi: 10.1016/s0735-1097(87)80471-0. [DOI] [PubMed] [Google Scholar]
- Jenkins O., Booth J. D., Minor P. D., Almond J. W. The complete nucleotide sequence of coxsackievirus B4 and its comparison to other members of the Picornaviridae. J Gen Virol. 1987 Jul;68(Pt 7):1835–1848. doi: 10.1099/0022-1317-68-7-1835. [DOI] [PubMed] [Google Scholar]
- Jin O., Sole M. J., Butany J. W., Chia W. K., McLaughlin P. R., Liu P., Liew C. C. Detection of enterovirus RNA in myocardial biopsies from patients with myocarditis and cardiomyopathy using gene amplification by polymerase chain reaction. Circulation. 1990 Jul;82(1):8–16. doi: 10.1161/01.cir.82.1.8. [DOI] [PubMed] [Google Scholar]
- Kandolf R., Ameis D., Kirschner P., Canu A., Hofschneider P. H. In situ detection of enteroviral genomes in myocardial cells by nucleic acid hybridization: an approach to the diagnosis of viral heart disease. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6272–6276. doi: 10.1073/pnas.84.17.6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler S., Galili N., Sklar J. L., Donlon T. A., Blume K. G., Cleary M. L. Expression of bcr-abl fusion transcripts following bone marrow transplantation for Philadelphia chromosome-positive leukemia. Leukemia. 1990 Aug;4(8):541–547. [PubMed] [Google Scholar]
- Kwok S., Mack D. H., Mullis K. B., Poiesz B., Ehrlich G., Blair D., Friedman-Kien A., Sninsky J. J. Identification of human immunodeficiency virus sequences by using in vitro enzymatic amplification and oligomer cleavage detection. J Virol. 1987 May;61(5):1690–1694. doi: 10.1128/jvi.61.5.1690-1694.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larzul D., Guigue F., Sninsky J. J., Mack D. H., Bréchot C., Guesdon J. L. Detection of hepatitis B virus sequences in serum by using in vitro enzymatic amplification. J Virol Methods. 1988 Jul;20(3):227–237. doi: 10.1016/0166-0934(88)90126-7. [DOI] [PubMed] [Google Scholar]
- Lerner A. M., Wilson F. M. Virus myocardiopathy. Prog Med Virol. 1973;15:63–91. [PubMed] [Google Scholar]
- Li H. H., Gyllensten U. B., Cui X. F., Saiki R. K., Erlich H. A., Arnheim N. Amplification and analysis of DNA sequences in single human sperm and diploid cells. Nature. 1988 Sep 29;335(6189):414–417. doi: 10.1038/335414a0. [DOI] [PubMed] [Google Scholar]
- Lindberg A. M., Stålhandske P. O., Pettersson U. Genome of coxsackievirus B3. Virology. 1987 Jan;156(1):50–63. doi: 10.1016/0042-6822(87)90435-1. [DOI] [PubMed] [Google Scholar]
- Ngan B. Y., Nourse J., Cleary M. L. Detection of chromosomal translocation t(14;18) within the minor cluster region of bcl-2 by polymerase chain reaction and direct genomic sequencing of the enzymatically amplified DNA in follicular lymphomas. Blood. 1989 May 15;73(7):1759–1762. [PubMed] [Google Scholar]
- Pincus S. E., Diamond D. C., Emini E. A., Wimmer E. Guanidine-selected mutants of poliovirus: mapping of point mutations to polypeptide 2C. J Virol. 1986 Feb;57(2):638–646. doi: 10.1128/jvi.57.2.638-646.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts W. C., Ferrans V. J. Pathologic anatomy of the cardiomyopathies. Idiopathic dilated and hypertrophic types, infiltrative types, and endomyocardial disease with and without eosinophilia. Hum Pathol. 1975 May;6(3):287–342. [PubMed] [Google Scholar]
- Shibata D. K., Arnheim N., Martin W. J. Detection of human papilloma virus in paraffin-embedded tissue using the polymerase chain reaction. J Exp Med. 1988 Jan 1;167(1):225–230. doi: 10.1084/jem.167.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazelaar H. D., Billingham M. E. Leukocytic infiltrates in idiopathic dilated cardiomyopathy. A source of confusion with active myocarditis. Am J Surg Pathol. 1986 Jun;10(6):405–412. doi: 10.1097/00000478-198606000-00005. [DOI] [PubMed] [Google Scholar]
- Weller P., Jeffreys A. J., Wilson V., Blanchetot A. Organization of the human myoglobin gene. EMBO J. 1984 Feb;3(2):439–446. doi: 10.1002/j.1460-2075.1984.tb01825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]