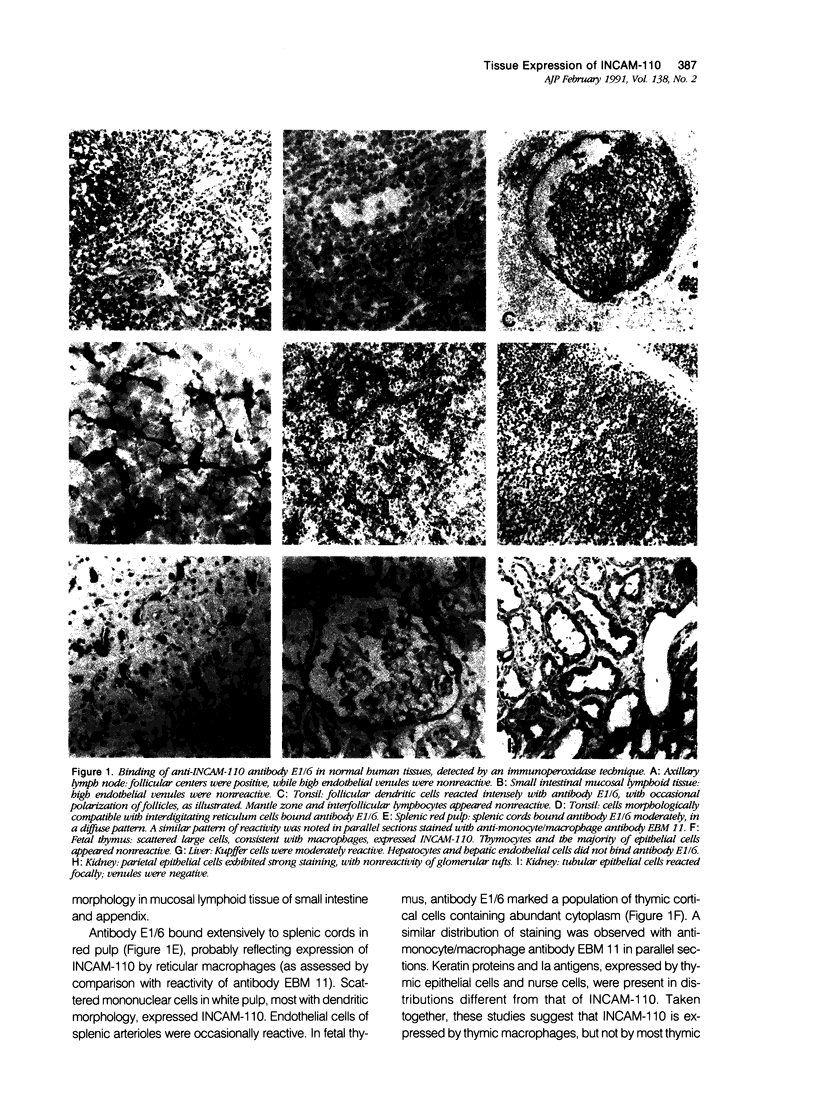
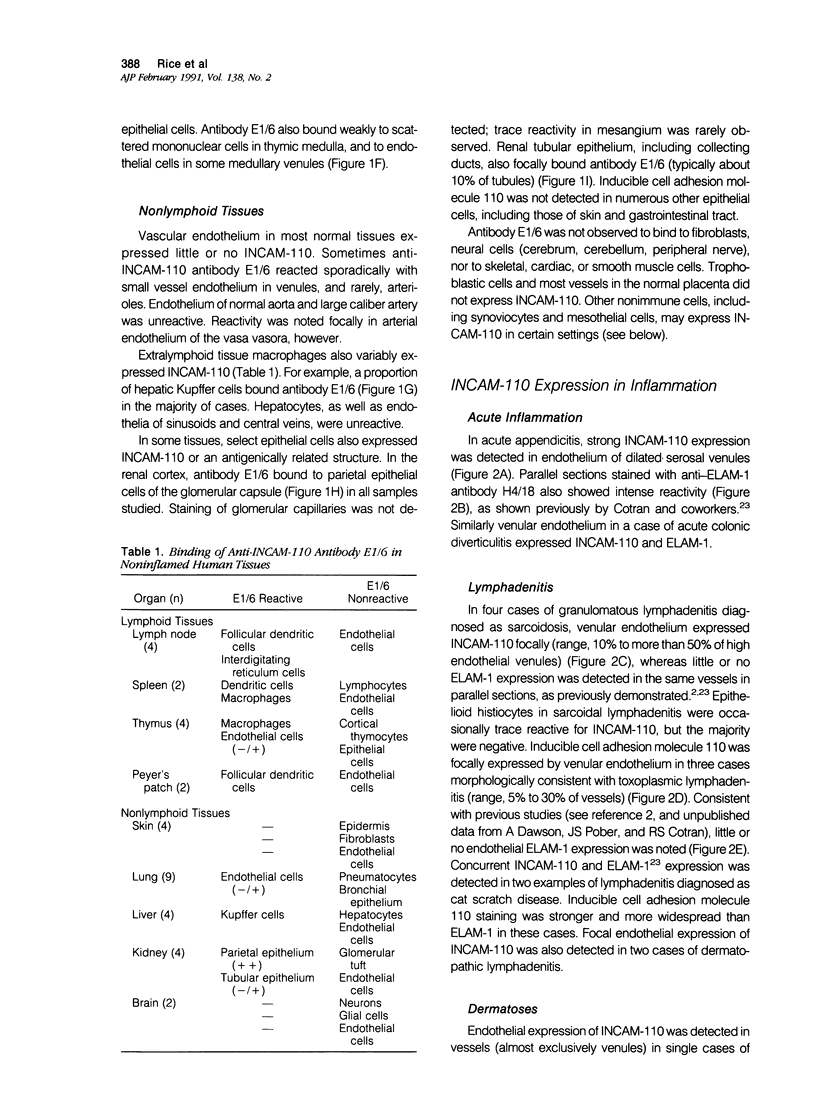
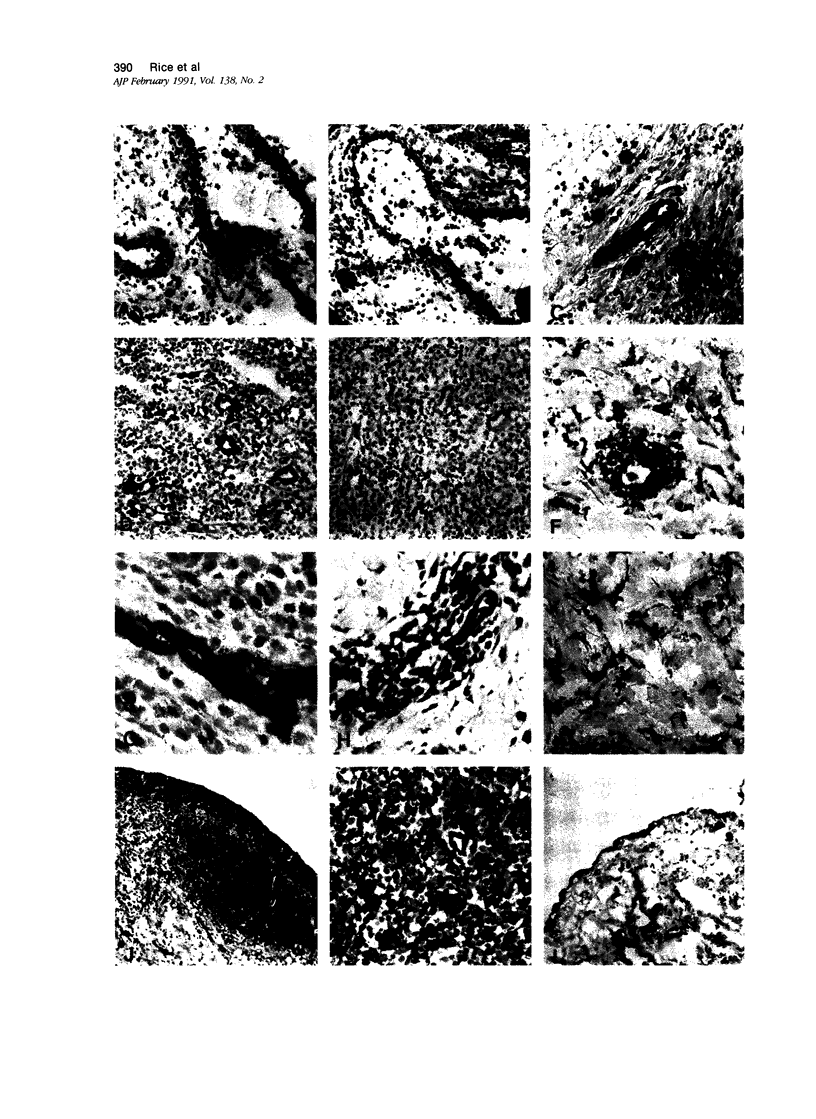
Abstract
Inducible cell adhesion molecule 110 (INCAM-110), is a 110-kd adhesion receptor for lymphocytes and monocytes identified on cytokine-activated endothelium. Using immunoperoxidase techniques, little or no INCAM-110 was detected on endothelium in normal human tissues. In contrast, INCAM-110 was expressed in postcapillary venules in a variety of active inflammatory processes. In acute appendicitis, INCAM-110 was found coincident with strong expression of endothelial leukocyte adhesion molecule 1 (ELAM-1), a cytokine-inducible molecule that functions in neutrophil adhesion. However, in certain chronic inflammatory processes (eg, sarcoidosis), INCAM-110 was observed without simultaneous ELAM-1 expression. Anti-INCAM-110 antibody E1/6 also marked several extravascular cell types, including lymphoid dendritic cells, some tissue macrophages, synovial lining cells, and reactive mesothelial cells. These data suggest a role for endothelial INCAM-110 in the pathophysiology of both acute and chronic inflammatory reactions. Furthermore INCAM-110 may function as an adhesion molecule for mononuclear leukocytes in a variety of extravascular sites.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. C., Schmalsteig F. C., Finegold M. J., Hughes B. J., Rothlein R., Miller L. J., Kohl S., Tosi M. F., Jacobs R. L., Waldrop T. C. The severe and moderate phenotypes of heritable Mac-1, LFA-1 deficiency: their quantitative definition and relation to leukocyte dysfunction and clinical features. J Infect Dis. 1985 Oct;152(4):668–689. doi: 10.1093/infdis/152.4.668. [DOI] [PubMed] [Google Scholar]
- Arnaout M. A., Lanier L. L., Faller D. V. Relative contribution of the leukocyte molecules Mo1, LFA-1, and p150,95 (LeuM5) in adhesion of granulocytes and monocytes to vascular endothelium is tissue- and stimulus-specific. J Cell Physiol. 1988 Nov;137(2):305–309. doi: 10.1002/jcp.1041370214. [DOI] [PubMed] [Google Scholar]
- Averbook B. J., Yamamoto R. S., Ulich T. R., Jeffes E. W., Masunaka I., Granger G. A. Purified native and recombinant human alpha lymphotoxin [tumor necrosis factor (TNF)-beta] induces inflammatory reactions in normal skin. J Clin Immunol. 1987 Jul;7(4):333–340. doi: 10.1007/BF00915556. [DOI] [PubMed] [Google Scholar]
- Beutler B., Cerami A. Cachectin and tumour necrosis factor as two sides of the same biological coin. Nature. 1986 Apr 17;320(6063):584–588. doi: 10.1038/320584a0. [DOI] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Mendrick D. L., Cotran R. S., Gimbrone M. A., Jr Identification of an inducible endothelial-leukocyte adhesion molecule. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9238–9242. doi: 10.1073/pnas.84.24.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Wheeler M. E., Cotran R. S., Gimbrone M. A., Jr Interleukin 1 acts on cultured human vascular endothelium to increase the adhesion of polymorphonuclear leukocytes, monocytes, and related leukocyte cell lines. J Clin Invest. 1985 Nov;76(5):2003–2011. doi: 10.1172/JCI112200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P., Stengelin S., Gimbrone M. A., Jr, Seed B. Endothelial leukocyte adhesion molecule 1: an inducible receptor for neutrophils related to complement regulatory proteins and lectins. Science. 1989 Mar 3;243(4895):1160–1165. doi: 10.1126/science.2466335. [DOI] [PubMed] [Google Scholar]
- Cavender D. E., Haskard D. O., Joseph B., Ziff M. Interleukin 1 increases the binding of human B and T lymphocytes to endothelial cell monolayers. J Immunol. 1986 Jan;136(1):203–207. [PubMed] [Google Scholar]
- Cotran R. S. American Association of Pathologists president's address. New roles for the endothelium in inflammation and immunity. Am J Pathol. 1987 Dec;129(3):407–413. [PMC free article] [PubMed] [Google Scholar]
- Cotran R. S., Gimbrone M. A., Jr, Bevilacqua M. P., Mendrick D. L., Pober J. S. Induction and detection of a human endothelial activation antigen in vivo. J Exp Med. 1986 Aug 1;164(2):661–666. doi: 10.1084/jem.164.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cybulsky M. I., Colditz I. G., Movat H. Z. The role of interleukin-1 in neutrophil leukocyte emigration induced by endotoxin. Am J Pathol. 1986 Sep;124(3):367–372. [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. Biology of interleukin 1. FASEB J. 1988 Feb;2(2):108–115. [PubMed] [Google Scholar]
- Dustin M. L., Rothlein R., Bhan A. K., Dinarello C. A., Springer T. A. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1). J Immunol. 1986 Jul 1;137(1):245–254. [PubMed] [Google Scholar]
- Dustin M. L., Singer K. H., Tuck D. T., Springer T. A. Adhesion of T lymphoblasts to epidermal keratinocytes is regulated by interferon gamma and is mediated by intercellular adhesion molecule 1 (ICAM-1). J Exp Med. 1988 Apr 1;167(4):1323–1340. doi: 10.1084/jem.167.4.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin M. L., Springer T. A. Lymphocyte function-associated antigen-1 (LFA-1) interaction with intercellular adhesion molecule-1 (ICAM-1) is one of at least three mechanisms for lymphocyte adhesion to cultured endothelial cells. J Cell Biol. 1988 Jul;107(1):321–331. doi: 10.1083/jcb.107.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elices M. J., Osborn L., Takada Y., Crouse C., Luhowskyj S., Hemler M. E., Lobb R. R. VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA-4/fibronectin binding site. Cell. 1990 Feb 23;60(4):577–584. doi: 10.1016/0092-8674(90)90661-w. [DOI] [PubMed] [Google Scholar]
- Freedman A. S., Munro J. M., Rice G. E., Bevilacqua M. P., Morimoto C., McIntyre B. W., Rhynhart K., Pober J. S., Nadler L. M. Adhesion of human B cells to germinal centers in vitro involves VLA-4 and INCAM-110. Science. 1990 Aug 31;249(4972):1030–1033. doi: 10.1126/science.1697696. [DOI] [PubMed] [Google Scholar]
- Gamble J. R., Harlan J. M., Klebanoff S. J., Vadas M. A. Stimulation of the adherence of neutrophils to umbilical vein endothelium by human recombinant tumor necrosis factor. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8667–8671. doi: 10.1073/pnas.82.24.8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths C. E., Nickoloff B. J. Keratinocyte intercellular adhesion molecule-1 (ICAM-1) expression precedes dermal T lymphocytic infiltration in allergic contact dermatitis (Rhus dermatitis). Am J Pathol. 1989 Dec;135(6):1045–1053. [PMC free article] [PubMed] [Google Scholar]
- Holzmann B., McIntyre B. W., Weissman I. L. Identification of a murine Peyer's patch--specific lymphocyte homing receptor as an integrin molecule with an alpha chain homologous to human VLA-4 alpha. Cell. 1989 Jan 13;56(1):37–46. doi: 10.1016/0092-8674(89)90981-1. [DOI] [PubMed] [Google Scholar]
- Inaba K., Romani N., Steinman R. M. An antigen-independent contact mechanism as an early step in T cell-proliferative responses to dendritic cells. J Exp Med. 1989 Aug 1;170(2):527–542. doi: 10.1084/jem.170.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K., Steinman R. M. Protein-specific helper T-lymphocyte formation initiated by dendritic cells. Science. 1985 Aug 2;229(4712):475–479. doi: 10.1126/science.3160115. [DOI] [PubMed] [Google Scholar]
- Jalkanen S., Steere A. C., Fox R. I., Butcher E. C. A distinct endothelial cell recognition system that controls lymphocyte traffic into inflamed synovium. Science. 1986 Aug 1;233(4763):556–558. doi: 10.1126/science.3726548. [DOI] [PubMed] [Google Scholar]
- Luscinskas F. W., Brock A. F., Arnaout M. A., Gimbrone M. A., Jr Endothelial-leukocyte adhesion molecule-1-dependent and leukocyte (CD11/CD18)-dependent mechanisms contribute to polymorphonuclear leukocyte adhesion to cytokine-activated human vascular endothelium. J Immunol. 1989 Apr 1;142(7):2257–2263. [PubMed] [Google Scholar]
- Makgoba M. W., Sanders M. E., Ginther Luce G. E., Dustin M. L., Springer T. A., Clark E. A., Mannoni P., Shaw S. ICAM-1 a ligand for LFA-1-dependent adhesion of B, T and myeloid cells. Nature. 1988 Jan 7;331(6151):86–88. doi: 10.1038/331086a0. [DOI] [PubMed] [Google Scholar]
- Marlin S. D., Springer T. A. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1). Cell. 1987 Dec 4;51(5):813–819. doi: 10.1016/0092-8674(87)90104-8. [DOI] [PubMed] [Google Scholar]
- Movat H. Z., Burrowes C. E., Cybulsky M. I., Dinarello C. A. Acute inflammation and a Shwartzman-like reaction induced by interleukin-1 and tumor necrosis factor. Synergistic action of the cytokines in the induction of inflammation and microvascular injury. Am J Pathol. 1987 Dec;129(3):463–476. [PMC free article] [PubMed] [Google Scholar]
- Munro J. M., Pober J. S., Cotran R. S. Tumor necrosis factor and interferon-gamma induce distinct patterns of endothelial activation and associated leukocyte accumulation in skin of Papio anubis. Am J Pathol. 1989 Jul;135(1):121–133. [PMC free article] [PubMed] [Google Scholar]
- Old L. J. Tumor necrosis factor (TNF). Science. 1985 Nov 8;230(4726):630–632. doi: 10.1126/science.2413547. [DOI] [PubMed] [Google Scholar]
- Osborn L., Hession C., Tizard R., Vassallo C., Luhowskyj S., Chi-Rosso G., Lobb R. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell. 1989 Dec 22;59(6):1203–1211. doi: 10.1016/0092-8674(89)90775-7. [DOI] [PubMed] [Google Scholar]
- Pober J. S. Warner-Lambert/Parke-Davis award lecture. Cytokine-mediated activation of vascular endothelium. Physiology and pathology. Am J Pathol. 1988 Dec;133(3):426–433. [PMC free article] [PubMed] [Google Scholar]
- Rice G. E., Bevilacqua M. P. An inducible endothelial cell surface glycoprotein mediates melanoma adhesion. Science. 1989 Dec 8;246(4935):1303–1306. doi: 10.1126/science.2588007. [DOI] [PubMed] [Google Scholar]
- Rice G. E., Munro J. M., Bevilacqua M. P. Inducible cell adhesion molecule 110 (INCAM-110) is an endothelial receptor for lymphocytes. A CD11/CD18-independent adhesion mechanism. J Exp Med. 1990 Apr 1;171(4):1369–1374. doi: 10.1084/jem.171.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum J. T., Howes E. L., Jr, Rubin R. M., Samples J. R. Ocular inflammatory effects of intravitreally-injected tumor necrosis factor. Am J Pathol. 1988 Oct;133(1):47–53. [PMC free article] [PubMed] [Google Scholar]
- Rothlein R., Dustin M. L., Marlin S. D., Springer T. A. A human intercellular adhesion molecule (ICAM-1) distinct from LFA-1. J Immunol. 1986 Aug 15;137(4):1270–1274. [PubMed] [Google Scholar]
- Schleimer R. P., Rutledge B. K. Cultured human vascular endothelial cells acquire adhesiveness for neutrophils after stimulation with interleukin 1, endotoxin, and tumor-promoting phorbol diesters. J Immunol. 1986 Jan;136(2):649–654. [PubMed] [Google Scholar]
- Simmons D., Makgoba M. W., Seed B. ICAM, an adhesion ligand of LFA-1, is homologous to the neural cell adhesion molecule NCAM. Nature. 1988 Feb 18;331(6157):624–627. doi: 10.1038/331624a0. [DOI] [PubMed] [Google Scholar]
- Smith C. W., Marlin S. D., Rothlein R., Toman C., Anderson D. C. Cooperative interactions of LFA-1 and Mac-1 with intercellular adhesion molecule-1 in facilitating adherence and transendothelial migration of human neutrophils in vitro. J Clin Invest. 1989 Jun;83(6):2008–2017. doi: 10.1172/JCI114111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. W., Rothlein R., Hughes B. J., Mariscalco M. M., Rudloff H. E., Schmalstieg F. C., Anderson D. C. Recognition of an endothelial determinant for CD 18-dependent human neutrophil adherence and transendothelial migration. J Clin Invest. 1988 Nov;82(5):1746–1756. doi: 10.1172/JCI113788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staunton D. E., Marlin S. D., Stratowa C., Dustin M. L., Springer T. A. Primary structure of ICAM-1 demonstrates interaction between members of the immunoglobulin and integrin supergene families. Cell. 1988 Mar 25;52(6):925–933. doi: 10.1016/0092-8674(88)90434-5. [DOI] [PubMed] [Google Scholar]
- Stoolman L. M. Adhesion molecules controlling lymphocyte migration. Cell. 1989 Mar 24;56(6):907–910. doi: 10.1016/0092-8674(89)90620-x. [DOI] [PubMed] [Google Scholar]
- Streeter P. R., Berg E. L., Rouse B. T., Bargatze R. F., Butcher E. C. A tissue-specific endothelial cell molecule involved in lymphocyte homing. Nature. 1988 Jan 7;331(6151):41–46. doi: 10.1038/331041a0. [DOI] [PubMed] [Google Scholar]
- Streeter P. R., Rouse B. T., Butcher E. C. Immunohistologic and functional characterization of a vascular addressin involved in lymphocyte homing into peripheral lymph nodes. J Cell Biol. 1988 Nov;107(5):1853–1862. doi: 10.1083/jcb.107.5.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R. Antigen-presenting function of the macrophage. Annu Rev Immunol. 1984;2:395–428. doi: 10.1146/annurev.iy.02.040184.002143. [DOI] [PubMed] [Google Scholar]
- Wankowicz Z., Megyeri P., Issekutz A. Synergy between tumour necrosis factor alpha and interleukin-1 in the induction of polymorphonuclear leukocyte migration during inflammation. J Leukoc Biol. 1988 Apr;43(4):349–356. doi: 10.1002/jlb.43.4.349. [DOI] [PubMed] [Google Scholar]