Abstract
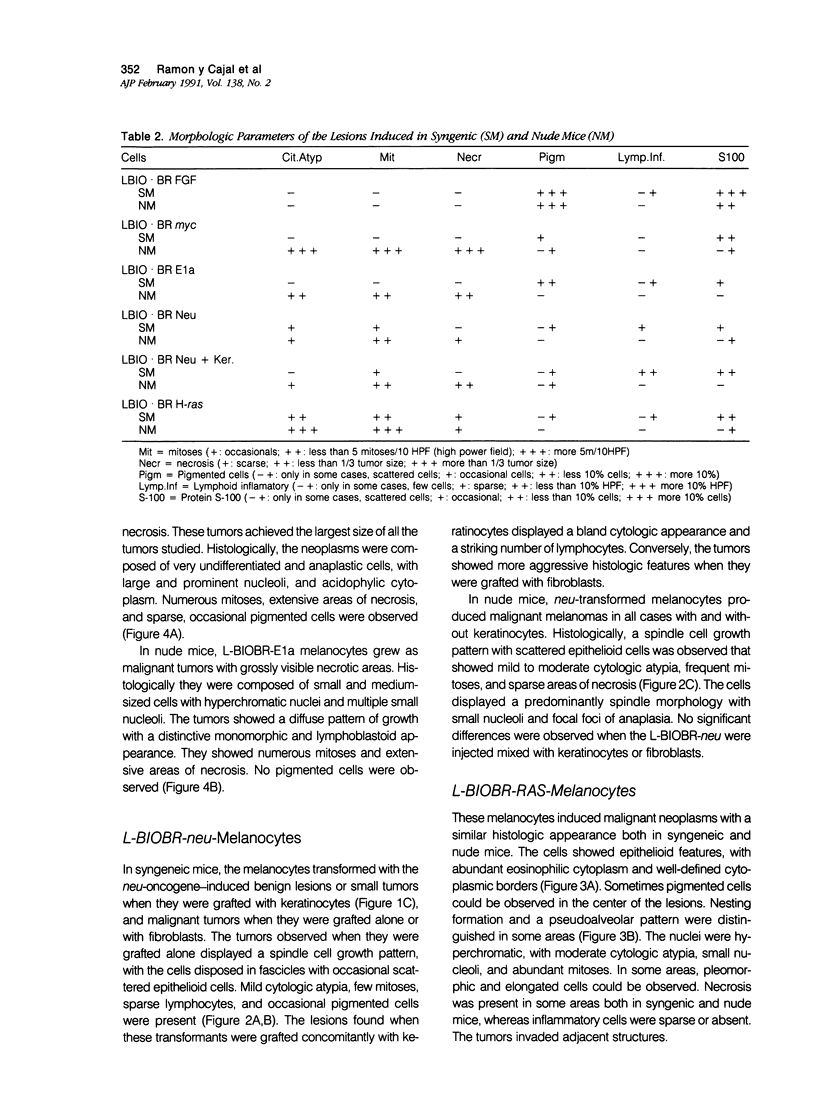
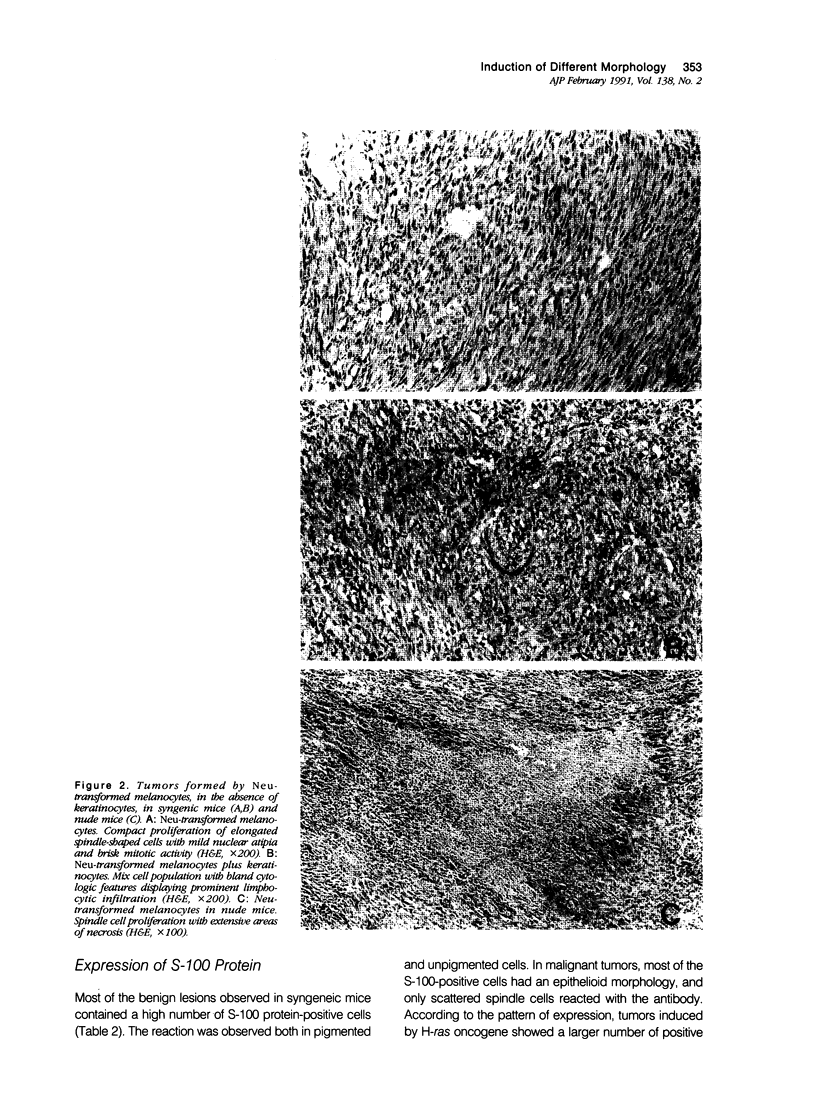
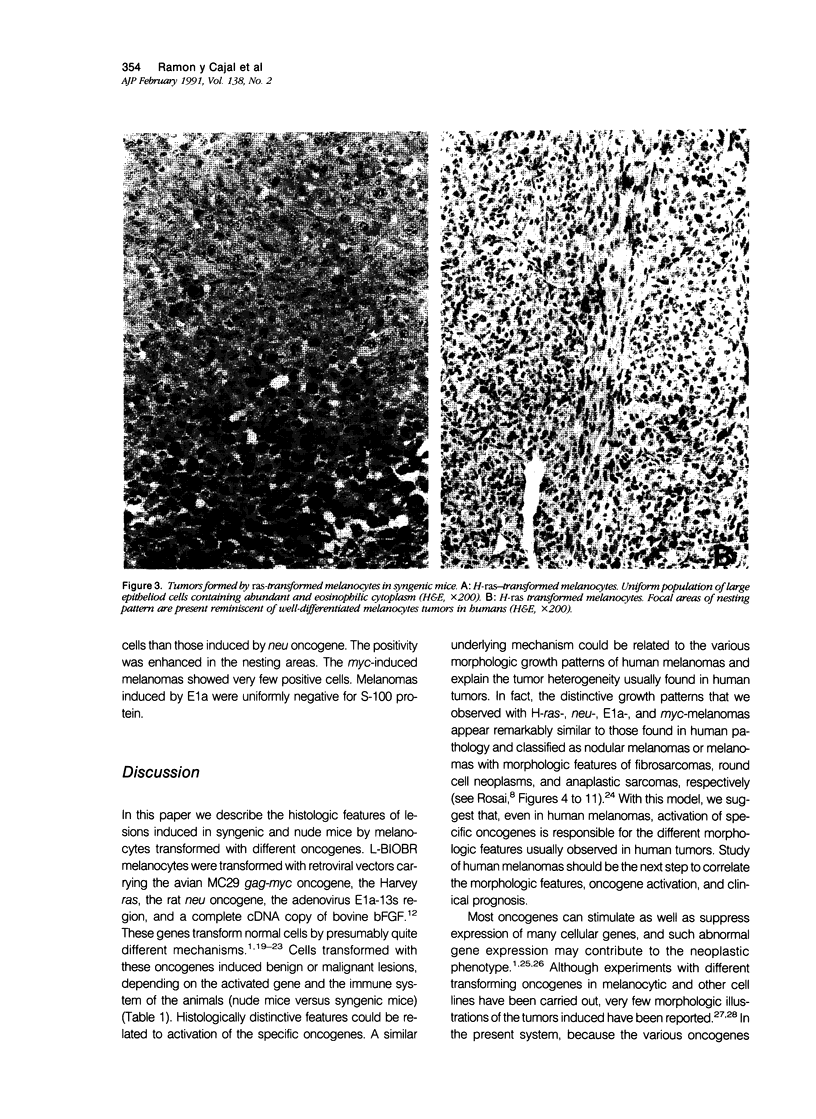
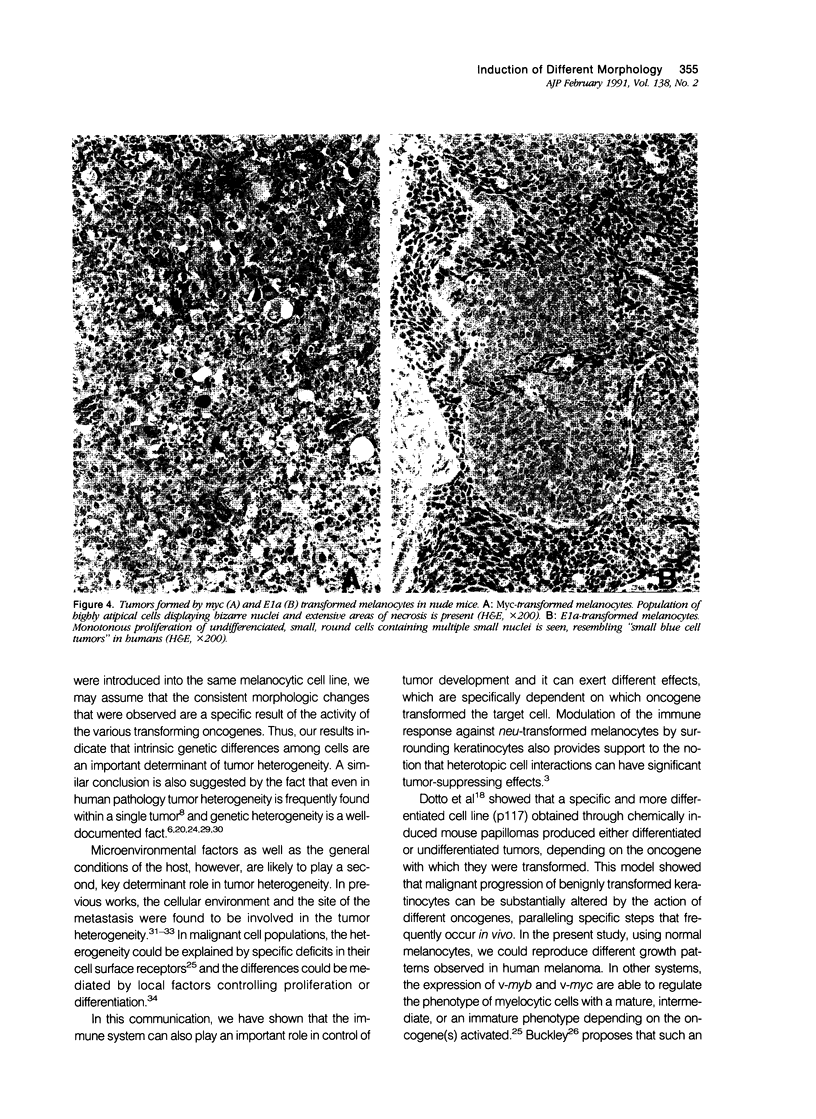
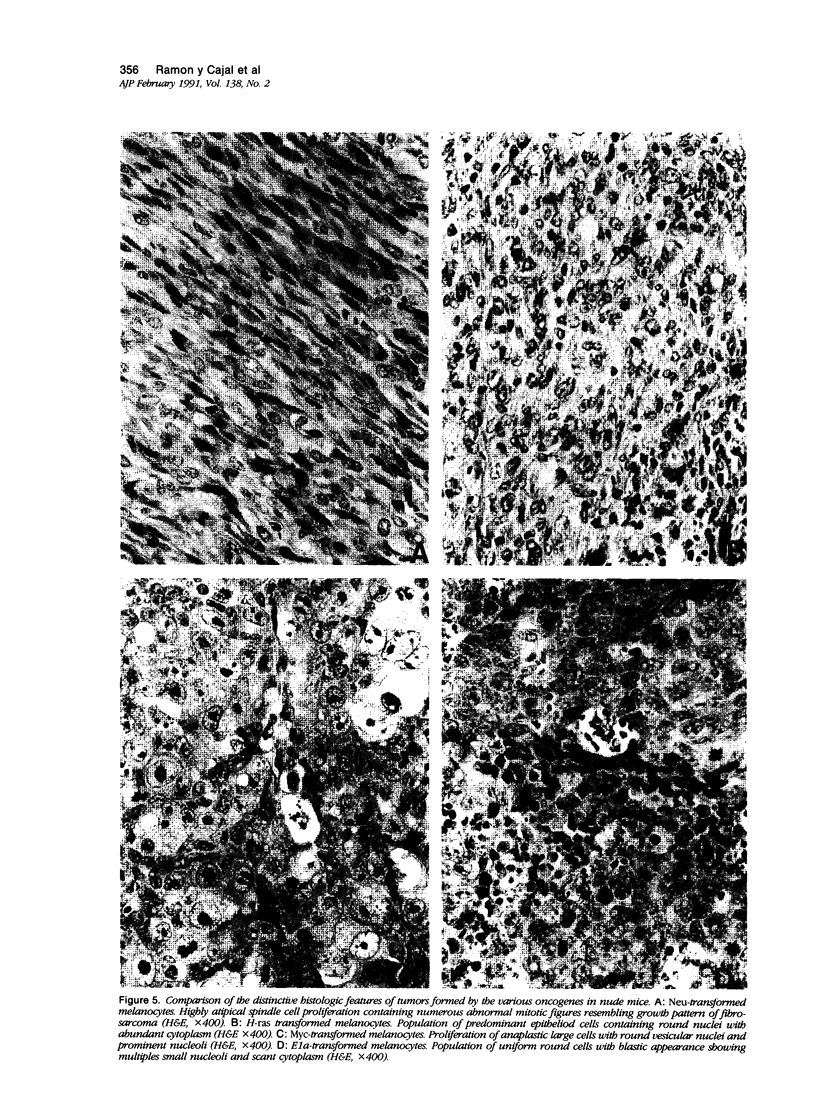
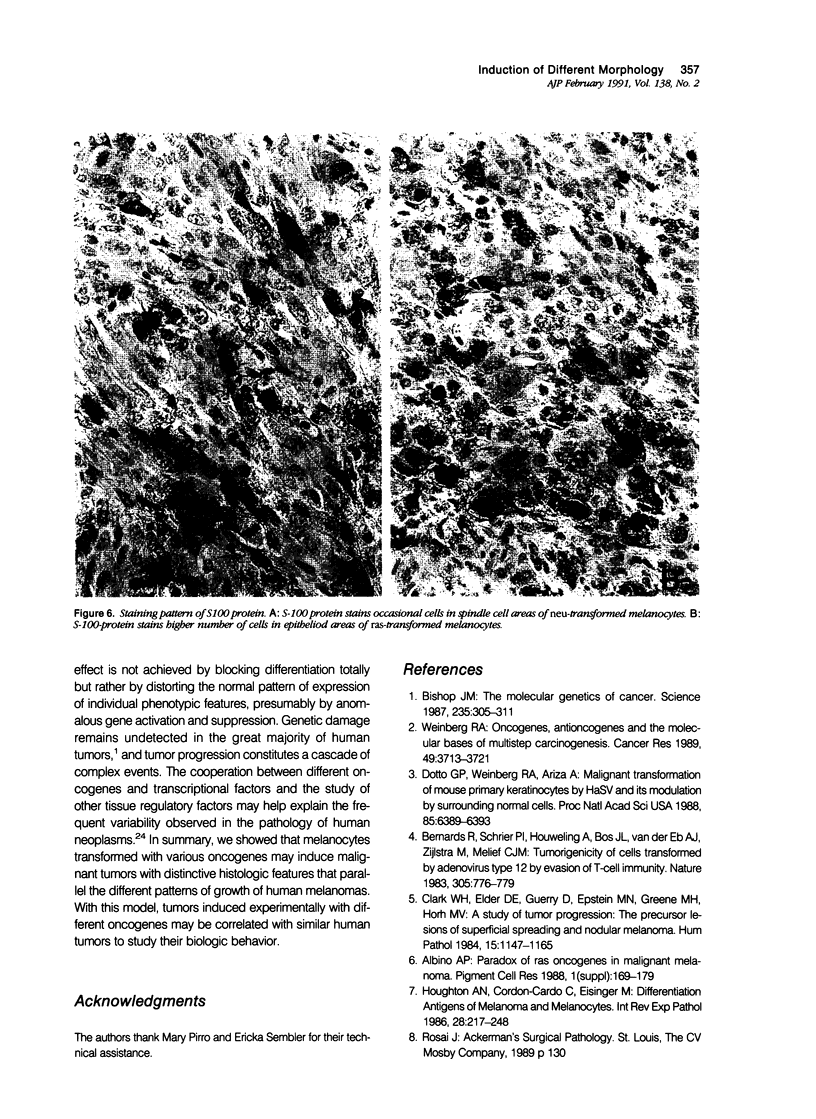
Malignant melanomas show a remarkable degree of heterogeneity because of different morphologic features, biologic behavior, and prognosis. In this communication, the authors attempted to correlate morphologic heterogeneity of melanomas with transformation by different activated oncogenes; they studied the histologic features of melanocytic lesions induced by murine melanocytes transformed by basic fibroblast growth factor (b-FGF-cDNA) or H-ras, neu, myc, and E1a oncogenes, and the lesions were compared with those observed in human pathology. Tumors formed after grafting onto syngenic mice or subcutaneous injections in nude mice were studied. In syngenic mice, benign melanocytic lesions reminiscent of intradermal nevus were observed with melanocytes transformed with b-FGF-cDNA, and myc and E1a oncogenes. Benign lesions were also formed by neu-transformed melanocytes when they were grafted concomitantly with keratinocytes, whereas malignant tumors were formed by the same cells when grafted alone or together with fibroblasts. In contrast, H-ras melanocytes always formed malignant tumors. In nude mice, b-FGF-transformed melanocytes induced benign lesions, whereas transformed melanocytes by the other oncogenes formed malignant tumors with distinctive and homogeneous morphologic features that depended on the transforming oncogene. Melanomas with either epithelioid cell, spindle cell, small round cell, and anaplastic cell growth patterns could be distinguished after transformation with H-ras, neu, E1a, and myc oncogenes, respectively. These various histologic types are analogous to those that may be observed in human melanomas, even within the same tumor. These studies suggest a possible molecular mechanism for tumor heterogeneity in which distinct oncogenes or oncogenelike activities can be activated in different tumors or discrete parts of the same tumor.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albino A. P., Lloyd K. O., Houghton A. N., Oettgen H. F., Old L. J. Heterogeneity in surface antigen and glycoprotein expression of cell lines derived from different melanoma metastases of the same patient. Implications for the study of tumor antigens. J Exp Med. 1981 Dec 1;154(6):1764–1778. doi: 10.1084/jem.154.6.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- Bargmann C. I., Weinberg R. A. Oncogenic activation of the neu-encoded receptor protein by point mutation and deletion. EMBO J. 1988 Jul;7(7):2043–2052. doi: 10.1002/j.1460-2075.1988.tb03044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernards R., Schrier P. I., Houweling A., Bos J. L., van der Eb A. J., Zijlstra M., Melief C. J. Tumorigenicity of cells transformed by adenovirus type 12 by evasion of T-cell immunity. 1983 Oct 27-Nov 2Nature. 305(5937):776–779. doi: 10.1038/305776a0. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. The molecular genetics of cancer. Science. 1987 Jan 16;235(4786):305–311. doi: 10.1126/science.3541204. [DOI] [PubMed] [Google Scholar]
- Bos J. L. ras oncogenes in human cancer: a review. Cancer Res. 1989 Sep 1;49(17):4682–4689. [PubMed] [Google Scholar]
- Buckley I. Oncogenes and the nature of malignancy. Adv Cancer Res. 1988;50:71–94. doi: 10.1016/s0065-230x(08)60435-2. [DOI] [PubMed] [Google Scholar]
- Clark W. H., Jr, Elder D. E., Guerry D., 4th, Epstein M. N., Greene M. H., Van Horn M. A study of tumor progression: the precursor lesions of superficial spreading and nodular melanoma. Hum Pathol. 1984 Dec;15(12):1147–1165. doi: 10.1016/s0046-8177(84)80310-x. [DOI] [PubMed] [Google Scholar]
- Dotto G. P., Moellmann G., Ghosh S., Edwards M., Halaban R. Transformation of murine melanocytes by basic fibroblast growth factor cDNA and oncogenes and selective suppression of the transformed phenotype in a reconstituted cutaneous environment. J Cell Biol. 1989 Dec;109(6 Pt 1):3115–3128. doi: 10.1083/jcb.109.6.3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotto G. P., O'Connell J., Patskan G., Conti C., Ariza A., Slaga T. J. Malignant progression of papilloma-derived keratinocytes: differential effects of the ras, neu, and p53 oncogenes. Mol Carcinog. 1988;1(3):171–179. doi: 10.1002/mc.2940010305. [DOI] [PubMed] [Google Scholar]
- Dotto G. P., Parada L. F., Weinberg R. A. Specific growth response of ras-transformed embryo fibroblasts to tumour promoters. Nature. 1985 Dec 5;318(6045):472–475. doi: 10.1038/318472a0. [DOI] [PubMed] [Google Scholar]
- Dotto G. P., Weinberg R. A., Ariza A. Malignant transformation of mouse primary keratinocytes by Harvey sarcoma virus and its modulation by surrounding normal cells. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6389–6393. doi: 10.1073/pnas.85.17.6389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downward J., Yarden Y., Mayes E., Scrace G., Totty N., Stockwell P., Ullrich A., Schlessinger J., Waterfield M. D. Close similarity of epidermal growth factor receptor and v-erb-B oncogene protein sequences. Nature. 1984 Feb 9;307(5951):521–527. doi: 10.1038/307521a0. [DOI] [PubMed] [Google Scholar]
- Gerhard D. S., Dracopoli N. C., Bale S. J., Houghton A. N., Watkins P., Payne C. E., Greene M. H., Housman D. E. Evidence against Ha-ras-1 involvement in sporadic and familial melanoma. Nature. 1987 Jan 1;325(6099):73–75. doi: 10.1038/325073a0. [DOI] [PubMed] [Google Scholar]
- Heppner G. H. Tumor heterogeneity. Cancer Res. 1984 Jun;44(6):2259–2265. [PubMed] [Google Scholar]
- Houghton A. N., Cordon-Cardo C., Eisinger M. Differentiation antigens of melanoma and melanocytes. Int Rev Exp Pathol. 1986;28:217–248. [PubMed] [Google Scholar]
- Kast W. M., Offringa R., Peters P. J., Voordouw A. C., Meloen R. H., van der Eb A. J., Melief C. J. Eradication of adenovirus E1-induced tumors by E1A-specific cytotoxic T lymphocytes. Cell. 1989 Nov 17;59(4):603–614. doi: 10.1016/0092-8674(89)90006-8. [DOI] [PubMed] [Google Scholar]
- Kaudewitz P., Braun-Falco O., Ernst M., Landthaler M., Stolz W., Gerdes J. Tumor cell growth fractions in human malignant melanomas and the correlation to histopathologic tumor grading. Am J Pathol. 1989 May;134(5):1063–1068. [PMC free article] [PubMed] [Google Scholar]
- Maize J. C. Primary cutaneous malignant melanoma. J Am Acad Dermatol. 1983 Jun;8(6):857–863. doi: 10.1016/s0190-9622(83)80017-6. [DOI] [PubMed] [Google Scholar]
- Price J. E., Tarin D., Fidler I. J. Influence of organ microenvironment on pigmentation of a metastatic murine melanoma. Cancer Res. 1988 Apr 15;48(8):2258–2264. [PubMed] [Google Scholar]
- Roberts B. E., Miller J. S., Kimelman D., Cepko C. L., Lemischka I. R., Mulligan R. C. Individual adenovirus type 5 early region 1A gene products elicit distinct alterations of cellular morphology and gene expression. J Virol. 1985 Nov;56(2):404–413. doi: 10.1128/jvi.56.2.404-413.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symonds G., Klempnauer K. H., Snyder M., Moscovici G., Moscovici C., Bishop J. M. Coordinate regulation of myelomonocytic phenotype by v-myb and v-myc. Mol Cell Biol. 1986 May;6(5):1796–1802. doi: 10.1128/mcb.6.5.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura A., Halaban R., Moellmann G., Cowan J. M., Lerner M. R., Lerner A. B. Normal murine melanocytes in culture. In Vitro Cell Dev Biol. 1987 Jul;23(7):519–522. doi: 10.1007/BF02628423. [DOI] [PubMed] [Google Scholar]
- Van Der Esch E. P., Cascinelli N., Preda F., Morabito A., Bufalino R. Stage I melanoma of the skin: evaluation of prognosis according to histologic characteristics. Cancer. 1981 Oct 1;48(7):1668–1673. doi: 10.1002/1097-0142(19811001)48:7<1668::aid-cncr2820480732>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A. Oncogenes, antioncogenes, and the molecular bases of multistep carcinogenesis. Cancer Res. 1989 Jul 15;49(14):3713–3721. [PubMed] [Google Scholar]
- Whyte P., Buchkovich K. J., Horowitz J. M., Friend S. H., Raybuck M., Weinberg R. A., Harlow E. Association between an oncogene and an anti-oncogene: the adenovirus E1A proteins bind to the retinoblastoma gene product. Nature. 1988 Jul 14;334(6178):124–129. doi: 10.1038/334124a0. [DOI] [PubMed] [Google Scholar]
- Wilson R. E., Dooley T. P., Hart I. R. Induction of tumorigenicity and lack of in vitro growth requirement for 12-O-tetradecanoylphorbol-13-acetate by transfection of murine melanocytes with v-Ha-ras. Cancer Res. 1989 Feb 1;49(3):711–716. [PubMed] [Google Scholar]
- Worst P. K., Mackenzie I. C., Fusenig N. E. Reformation of organized epidermal structure by transplantation of suspensions and cultures of epidermal and dermal cells. Cell Tissue Res. 1982;225(1):65–77. doi: 10.1007/BF00216219. [DOI] [PubMed] [Google Scholar]
- Yasuda H., Kobayashi H., Ohkawara A., Kuzumaki N. Differential expression of ras oncogene products among the types of human melanomas and melanocytic nevi. J Invest Dermatol. 1989 Jul;93(1):54–59. doi: 10.1111/1523-1747.ep12277350. [DOI] [PubMed] [Google Scholar]
- van 't Veer L. J., Burgering B. M., Versteeg R., Boot A. J., Ruiter D. J., Osanto S., Schrier P. I., Bos J. L. N-ras mutations in human cutaneous melanoma from sun-exposed body sites. Mol Cell Biol. 1989 Jul;9(7):3114–3116. doi: 10.1128/mcb.9.7.3114. [DOI] [PMC free article] [PubMed] [Google Scholar]








