Abstract
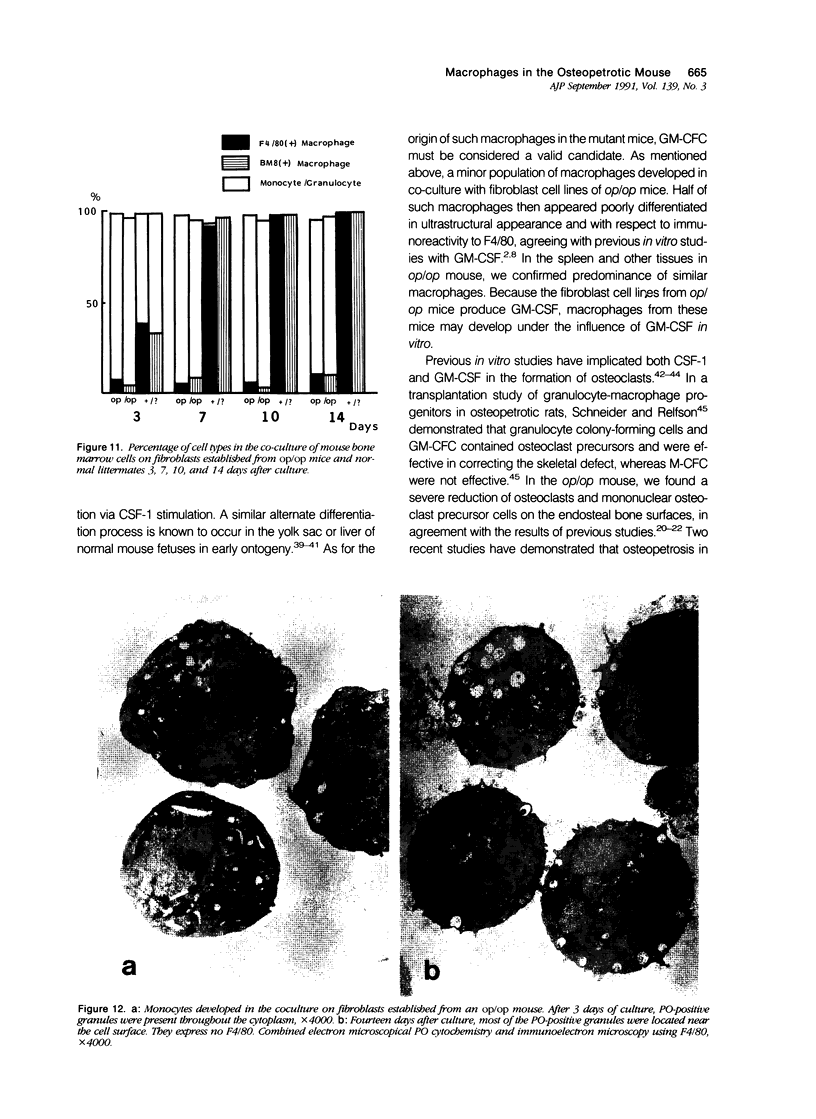
Examination of the op/op mouse disclosed marked reduction and abnormal differentiation of osteoclasts in the bones and of tissue-specific macrophages in various visceral organs and tissues. Most of these macrophages were immature as judged by ultrastructural criteria. In co-cultures of normal mouse bone marrow cells with fibroblast cell lines prepared from the lungs of the op/op mice, a defective differentiation of monocytes into macrophages was confirmed, supporting previous evidence that the fibroblast cell lines of the mutant mouse failed to produce functional macrophage colony-stimulating factor (M-CSF/CSF-1). In such co-cultures, however, a small number of macrophages apparently mature under the influence of granulocyte/macrophage colony-stimulating factor (GM-CSF) produced by the op/op fibroblast cell lines. In the mutant mice, the numbers of macrophages in the uterine wall and ovaries were severely reduced. Compared with the tissues of normal littermates, those of the mutants contained about 60% fewer macrophages in many tissues. This suggests that an M-CSF-independent population of macrophages is derived from granulocyte/macrophage-colony-forming cells (GM-CFC) or earlier hematopoietic progenitors.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arceci R. J., Shanahan F., Stanley E. R., Pollard J. W. Temporal expression and location of colony-stimulating factor 1 (CSF-1) and its receptor in the female reproductive tract are consistent with CSF-1-regulated placental development. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8818–8822. doi: 10.1073/pnas.86.22.8818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartocci A., Pollard J. W., Stanley E. R. Regulation of colony-stimulating factor 1 during pregnancy. J Exp Med. 1986 Sep 1;164(3):956–961. doi: 10.1084/jem.164.3.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers T. J., Loutit J. F. A functional assessment of macrophages from osteopetrotic mice. J Pathol. 1979 Oct;129(2):57–63. doi: 10.1002/path.1711290203. [DOI] [PubMed] [Google Scholar]
- Chambers T. J. Phagocytosis and trypsin-resistant glass adhesion by osteoclasts in culture. J Pathol. 1979 Feb;127(2):55–60. doi: 10.1002/path.1711270202. [DOI] [PubMed] [Google Scholar]
- Cohn Z. A. Activation of mononuclear phagocytes: fact, fancy, and future. J Immunol. 1978 Sep;121(3):813–816. [PubMed] [Google Scholar]
- Falk L. A., Vogel S. N. Comparison of bone marrow progenitors responsive to granulocyte-macrophage colony stimulating factor and macrophage colony stimulating factor-1. J Leukoc Biol. 1988 Feb;43(2):148–157. doi: 10.1002/jlb.43.2.148. [DOI] [PubMed] [Google Scholar]
- Falk L. A., Vogel S. N. Differential production of IFN-alpha/beta by CSF-1- and GM-CSF-derived macrophages. J Leukoc Biol. 1990 Jul;48(1):43–49. doi: 10.1002/jlb.48.1.43. [DOI] [PubMed] [Google Scholar]
- Felix R., Cecchini M. G., Fleisch H. Macrophage colony stimulating factor restores in vivo bone resorption in the op/op osteopetrotic mouse. Endocrinology. 1990 Nov;127(5):2592–2594. doi: 10.1210/endo-127-5-2592. [DOI] [PubMed] [Google Scholar]
- Felix R., Cecchini M. G., Hofstetter W., Elford P. R., Stutzer A., Fleisch H. Impairment of macrophage colony-stimulating factor production and lack of resident bone marrow macrophages in the osteopetrotic op/op mouse. J Bone Miner Res. 1990 Jul;5(7):781–789. doi: 10.1002/jbmr.5650050716. [DOI] [PubMed] [Google Scholar]
- Geissler K., Harrington M., Srivastava C., Leemhuis T., Tricot G., Broxmeyer H. E. Effects of recombinant human colony stimulating factors (CSF) (granulocyte-macrophage CSF, granulocyte CSF, and CSF-1) on human monocyte/macrophage differentiation. J Immunol. 1989 Jul 1;143(1):140–146. [PubMed] [Google Scholar]
- Hammarström L. E., Hanker J. S., Toverud S. U. Cellular differences in acid phosphatase isoenzymes in bone and teeth. Clin Orthop Relat Res. 1971;78:151–167. doi: 10.1097/00003086-197107000-00012. [DOI] [PubMed] [Google Scholar]
- Hattersley G., Chambers T. J. Effects of interleukin 3 and of granulocyte-macrophage and macrophage colony stimulating factors on osteoclast differentiation from mouse hemopoietic tissue. J Cell Physiol. 1990 Jan;142(1):201–209. doi: 10.1002/jcp.1041420125. [DOI] [PubMed] [Google Scholar]
- Hume D. A., Loutit J. F., Gordon S. The mononuclear phagocyte system of the mouse defined by immunohistochemical localization of antigen F4/80: macrophages of bone and associated connective tissue. J Cell Sci. 1984 Mar;66:189–194. doi: 10.1242/jcs.66.1.189. [DOI] [PubMed] [Google Scholar]
- Hume D. A., Robinson A. P., MacPherson G. G., Gordon S. The mononuclear phagocyte system of the mouse defined by immunohistochemical localization of antigen F4/80. Relationship between macrophages, Langerhans cells, reticular cells, and dendritic cells in lymphoid and hematopoietic organs. J Exp Med. 1983 Nov 1;158(5):1522–1536. doi: 10.1084/jem.158.5.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama H., Yamasaki A., Nose M., Niida S., Ohgame Y., Abe M., Kumegawa M., Suda T. Congenital osteoclast deficiency in osteopetrotic (op/op) mice is cured by injections of macrophage colony-stimulating factor. J Exp Med. 1991 Jan 1;173(1):269–272. doi: 10.1084/jem.173.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loutit J. F., Nisbet N. W. Resorption of bone. Lancet. 1979 Jul 7;2(8132):26–27. doi: 10.1016/s0140-6736(79)90186-7. [DOI] [PubMed] [Google Scholar]
- MacDonald B. R., Mundy G. R., Clark S., Wang E. A., Kuehl T. J., Stanley E. R., Roodman G. D. Effects of human recombinant CSF-GM and highly purified CSF-1 on the formation of multinucleated cells with osteoclast characteristics in long-term bone marrow cultures. J Bone Miner Res. 1986 Apr;1(2):227–233. doi: 10.1002/jbmr.5650010210. [DOI] [PubMed] [Google Scholar]
- Malorny U., Michels E., Sorg C. A monoclonal antibody against an antigen present on mouse macrophages and absent from monocytes. Cell Tissue Res. 1986;243(2):421–428. doi: 10.1007/BF00251059. [DOI] [PubMed] [Google Scholar]
- Marks S. C., Jr, Lane P. W. Osteopetrosis, a new recessive skeletal mutation on chromosome 12 of the mouse. J Hered. 1976 Jan-Feb;67(1):11–18. doi: 10.1093/oxfordjournals.jhered.a108657. [DOI] [PubMed] [Google Scholar]
- Marks S. C., Jr Morphological evidence of reduced bone resorption in osteopetrotic (op) mice. Am J Anat. 1982 Feb;163(2):157–167. doi: 10.1002/aja.1001630205. [DOI] [PubMed] [Google Scholar]
- Marks S. C., Jr, Seifert M. F., McGuire J. L. Congenitally osteopetrotic (oplop) mice are not cured by transplants of spleen or bone marrow cells from normal littermates. Metab Bone Dis Relat Res. 1984;5(4):183–186. doi: 10.1016/0221-8747(84)90027-4. [DOI] [PubMed] [Google Scholar]
- Morrissey P. J., Bressler L., Charrier K., Alpert A. Response of resident murine peritoneal macrophages to in vivo administration of granulocyte-macrophage colony-stimulating factor. J Immunol. 1988 Mar 15;140(6):1910–1915. [PubMed] [Google Scholar]
- Morrissey P. J., Grabstein K. H., Reed S. G., Conlon P. J. Granulocyte/macrophage colony stimulating factor. A potent activation signal for mature macrophages and monocytes. Int Arch Allergy Appl Immunol. 1989;88(1-2):40–45. [PubMed] [Google Scholar]
- Naito M., Takahashi K., Nishikawa S. Development, differentiation, and maturation of macrophages in the fetal mouse liver. J Leukoc Biol. 1990 Jul;48(1):27–37. doi: 10.1002/jlb.48.1.27. [DOI] [PubMed] [Google Scholar]
- Naito M., Yamamura F., Nishikawa S., Takahashi K. Development, differentiation, and maturation of fetal mouse yolk sac macrophages in cultures. J Leukoc Biol. 1989 Jul;46(1):1–10. doi: 10.1002/jlb.46.1.1. [DOI] [PubMed] [Google Scholar]
- Novak J. P., Skamene E., Gervais F. Quantitative model of mononuclear phagocyte lineage proliferation in murine bone marrow. J Leukoc Biol. 1989 Jul;46(1):25–33. doi: 10.1002/jlb.46.1.25. [DOI] [PubMed] [Google Scholar]
- Pennline K. J., Pellerito F., DaFonseca M., Monahan P., Siegel M. I., Smith S. R. Flow cytometric analysis of recombinant murine GM-CSF (rmuGM-CSF) induced changes in the distribution of specific cell populations in vivo. Cytometry. 1990;11(2):283–291. doi: 10.1002/cyto.990110209. [DOI] [PubMed] [Google Scholar]
- Pollard J. W., Bartocci A., Arceci R., Orlofsky A., Ladner M. B., Stanley E. R. Apparent role of the macrophage growth factor, CSF-1, in placental development. Nature. 1987 Dec 3;330(6147):484–486. doi: 10.1038/330484a0. [DOI] [PubMed] [Google Scholar]
- Rajavashisth T. B., Eng R., Shadduck R. K., Waheed A., Ben-Avram C. M., Shively J. E., Lusis A. J. Cloning and tissue-specific expression of mouse macrophage colony-stimulating factor mRNA. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1157–1161. doi: 10.1073/pnas.84.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regenstreif L. J., Rossant J. Expression of the c-fms proto-oncogene and of the cytokine, CSF-1, during mouse embryogenesis. Dev Biol. 1989 May;133(1):284–294. doi: 10.1016/0012-1606(89)90319-9. [DOI] [PubMed] [Google Scholar]
- Schneider G. B., Relfson M. The effects of transplantation of granulocyte-macrophage progenitors on bone resorption in osteopetrotic rats. J Bone Miner Res. 1988 Apr;3(2):225–232. doi: 10.1002/jbmr.5650030216. [DOI] [PubMed] [Google Scholar]
- Seifert M. F., Marks S. C., Jr Congenitally osteosclerotic (oc/oc) mice are resistant to cure by transplantation of bone marrow or spleen cells from normal littermates. Tissue Cell. 1987;19(1):29–37. doi: 10.1016/0040-8166(87)90054-1. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Yamamura F., Naito M. Differentiation, maturation, and proliferation of macrophages in the mouse yolk sac: a light-microscopic, enzyme-cytochemical, immunohistochemical, and ultrastructural study. J Leukoc Biol. 1989 Feb;45(2):87–96. doi: 10.1002/jlb.45.2.87. [DOI] [PubMed] [Google Scholar]
- Wiktor-Jedrzejczak W. W., Ahmed A., Szczylik C., Skelly R. R. Hematological characterization of congenital osteopetrosis in op/op mouse. Possible mechanism for abnormal macrophage differentiation. J Exp Med. 1982 Nov 1;156(5):1516–1527. doi: 10.1084/jem.156.5.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiktor-Jedrzejczak W., Bartocci A., Ferrante A. W., Jr, Ahmed-Ansari A., Sell K. W., Pollard J. W., Stanley E. R. Total absence of colony-stimulating factor 1 in the macrophage-deficient osteopetrotic (op/op) mouse. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4828–4832. doi: 10.1073/pnas.87.12.4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H., Hayashi S., Kunisada T., Ogawa M., Nishikawa S., Okamura H., Sudo T., Shultz L. D., Nishikawa S. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature. 1990 May 31;345(6274):442–444. doi: 10.1038/345442a0. [DOI] [PubMed] [Google Scholar]
- van Furth R., Cohn Z. A., Hirsch J. G., Humphrey J. H., Spector W. G., Langevoort H. L. The mononuclear phagocyte system: a new classification of macrophages, monocytes, and their precursor cells. Bull World Health Organ. 1972;46(6):845–852. [PMC free article] [PubMed] [Google Scholar]