Abstract
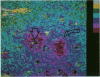
Amyloid is a component of the senile plaques that characterize one of the major neuropathologic changes in patients with Alzheimer's disease (AD). The sequence of events leading to the accumulation of amyloid precursors in senile plaques is unknown. In previous studies, the authors have shown that congophilic deposits in a subset of mature amyloid plaques are angiocentric. In this study, the authors used image analysis microspectroscopy and an antibody directed against a synthetic beta-protein (beta) or A4 sequence to examine the distribution patterns of this protein in serial sections from brains of patients with AD and in normal aged brains after quantitative immunohistochemistry. Image analysis of early primitive plaques disclosed two main patterns of early beta/A4 deposition, which consisted of neurocentric and angiocentric decreasing concentration gradients. In most instances, these gradients were not recognizable by the naked eye but appeared strikingly conspicuous after image subtraction and pseudocoloring. The described neurocentric gradients suggest that deposition of this protein, in at least some early primitive plaques, is related to neurons and possibly originates from these cells. The opposite viewpoint, i.e., that peripherally synthesized beta/A4 protein would 'sink in' toward neurons, is not supported because in very early plaques the highest immunoreactivity within the gradient was the neuronal body itself. A hypothesis is offered to reconcile the presence of both neurocentric and angiocentric depositions of these substances.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allsop D., Haga S. I., Haga C., Ikeda S. I., Mann D. M., Ishii T. Early senile plaques in Down's syndrome brains show a close relationship with cell bodies of neurons. Neuropathol Appl Neurobiol. 1989 Nov-Dec;15(6):531–542. doi: 10.1111/j.1365-2990.1989.tb01252.x. [DOI] [PubMed] [Google Scholar]
- Arai H., Sagi N., Noguchi I., Haga S., Ishii T., Makino Y., Kosaka K. An immunohistochemical study of beta-protein in Alzheimer-type dementia brains. J Neurol. 1989 May;236(4):214–217. doi: 10.1007/BF00314502. [DOI] [PubMed] [Google Scholar]
- Armstrong D. M., LeRoy S., Shields D., Terry R. D. Somatostatin-like immunoreactivity within neuritic plaques. Brain Res. 1985 Jul 8;338(1):71–79. doi: 10.1016/0006-8993(85)90249-5. [DOI] [PubMed] [Google Scholar]
- Cohen M. L., Golde T. E., Usiak M. F., Younkin L. H., Younkin S. G. In situ hybridization of nucleus basalis neurons shows increased beta-amyloid mRNA in Alzheimer disease. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1227–1231. doi: 10.1073/pnas.85.4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cras P., Kawai M., Siedlak S., Mulvihill P., Gambetti P., Lowery D., Gonzalez-DeWhitt P., Greenberg B., Perry G. Neuronal and microglial involvement in beta-amyloid protein deposition in Alzheimer's disease. Am J Pathol. 1990 Aug;137(2):241–246. [PMC free article] [PubMed] [Google Scholar]
- Dayan A. D. Quantitative histological studies on the aged human brain. I. Senile plaques and neurofibrillary tangles in "normal" patients. Acta Neuropathol. 1970;16(2):85–94. doi: 10.1007/BF00687663. [DOI] [PubMed] [Google Scholar]
- Delacourte A., Defossez A., Persuy P., Peers M. C. Observation of morphological relationships between angiopathic blood vessels and degenerative neurites in Alzheimer's disease. Virchows Arch A Pathol Anat Histopathol. 1987;411(3):199–204. doi: 10.1007/BF00735024. [DOI] [PubMed] [Google Scholar]
- Glenner G. G. Congophilic microangiopathy in the pathogenesis of Alzheimer's syndrome (presenile dementia). Med Hypotheses. 1979 Nov;5(11):1231–1236. doi: 10.1016/0306-9877(79)90005-7. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Henry J. H., Fujihara S. Congophilic angiopathy in the pathogenesis of Alzheimer's degeneration. Ann Pathol. 1981;1(2):120–129. [PubMed] [Google Scholar]
- Glenner G. G., Wong C. W. Alzheimer's disease and Down's syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun. 1984 Aug 16;122(3):1131–1135. doi: 10.1016/0006-291x(84)91209-9. [DOI] [PubMed] [Google Scholar]
- Gonatas N. K., Anderson W., Evangelista I. The contribution of altered synapses in the senile plaque: an electron microscopic study in Alzheimer's dementia. J Neuropathol Exp Neurol. 1967 Jan;26(1):25–39. doi: 10.1097/00005072-196701000-00003. [DOI] [PubMed] [Google Scholar]
- Hart M. N., Merz P., Bennett-Gray J., Menezes A. H., Goeken J. A., Schelper R. L., Wisniewski H. M. beta-amyloid protein of Alzheimer's disease is found in cerebral and spinal cord vascular malformations. Am J Pathol. 1988 Jul;132(1):167–172. [PMC free article] [PubMed] [Google Scholar]
- Higgins G. A., Lewis D. A., Bahmanyar S., Goldgaber D., Gajdusek D. C., Young W. G., Morrison J. H., Wilson M. C. Differential regulation of amyloid-beta-protein mRNA expression within hippocampal neuronal subpopulations in Alzheimer disease. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1297–1301. doi: 10.1073/pnas.85.4.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. The use of antiavidin antibody and avidin-biotin-peroxidase complex in immunoperoxidase technics. Am J Clin Pathol. 1981 Jun;75(6):816–821. doi: 10.1093/ajcp/75.6.816. [DOI] [PubMed] [Google Scholar]
- Itagaki S., McGeer P. L., Akiyama H., Zhu S., Selkoe D. Relationship of microglia and astrocytes to amyloid deposits of Alzheimer disease. J Neuroimmunol. 1989 Oct;24(3):173–182. doi: 10.1016/0165-5728(89)90115-x. [DOI] [PubMed] [Google Scholar]
- Joachim C. L., Mori H., Selkoe D. J. Amyloid beta-protein deposition in tissues other than brain in Alzheimer's disease. Nature. 1989 Sep 21;341(6239):226–230. doi: 10.1038/341226a0. [DOI] [PubMed] [Google Scholar]
- Johnson S. A., McNeill T., Cordell B., Finch C. E. Relation of neuronal APP-751/APP-695 mRNA ratio and neuritic plaque density in Alzheimer's disease. Science. 1990 May 18;248(4957):854–857. doi: 10.1126/science.2111579. [DOI] [PubMed] [Google Scholar]
- Kang J., Lemaire H. G., Unterbeck A., Salbaum J. M., Masters C. L., Grzeschik K. H., Multhaup G., Beyreuther K., Müller-Hill B. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987 Feb 19;325(6106):733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Khachaturian Z. S. Diagnosis of Alzheimer's disease. Arch Neurol. 1985 Nov;42(11):1097–1105. doi: 10.1001/archneur.1985.04060100083029. [DOI] [PubMed] [Google Scholar]
- Kitaguchi N., Takahashi Y., Tokushima Y., Shiojiri S., Ito H. Novel precursor of Alzheimer's disease amyloid protein shows protease inhibitory activity. Nature. 1988 Feb 11;331(6156):530–532. doi: 10.1038/331530a0. [DOI] [PubMed] [Google Scholar]
- Kosik K. S., Rogers J., Kowall N. W. Senile plaques are located between apical dendritic clusters. J Neuropathol Exp Neurol. 1987 Jan;46(1):1–11. doi: 10.1097/00005072-198701000-00001. [DOI] [PubMed] [Google Scholar]
- Masters C. L., Simms G., Weinman N. A., Multhaup G., McDonald B. L., Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattiace L. A., Davies P., Yen S. H., Dickson D. W. Microglia in cerebellar plaques in Alzheimer's disease. Acta Neuropathol. 1990;80(5):493–498. doi: 10.1007/BF00294609. [DOI] [PubMed] [Google Scholar]
- Miyakawa T., Shimoji A., Kuramoto R., Higuchi Y. The relationship between senile plaques and cerebral blood vessels in Alzheimer's disease and senile dementia. Morphological mechanism of senile plaque production. Virchows Arch B Cell Pathol Incl Mol Pathol. 1982 Aug;40(2):121–129. doi: 10.1007/BF02932857. [DOI] [PubMed] [Google Scholar]
- Morimatsu M., Hirai S., Muramatsu A., Yoshikawa M. Senile degenerative brain lesions and dementia. J Am Geriatr Soc. 1975 Sep;23(9):390–406. doi: 10.1111/j.1532-5415.1975.tb00425.x. [DOI] [PubMed] [Google Scholar]
- Neve R. L., Rogers J., Higgins G. A. The Alzheimer amyloid precursor-related transcript lacking the beta/A4 sequence is specifically increased in Alzheimer's disease brain. Neuron. 1990 Sep;5(3):329–338. doi: 10.1016/0896-6273(90)90169-g. [DOI] [PubMed] [Google Scholar]
- Pappolla M. A. Computerized image-analysis microspectroscopy of tissue sections. Arch Pathol Lab Med. 1988 Aug;112(8):787–790. [PubMed] [Google Scholar]
- Pappolla M. A. Image analysis microspectroscopy of senile plaque capillary amyloid in Alzheimer's disease. A preliminary study. Arch Pathol Lab Med. 1989 Aug;113(8):866–871. [PubMed] [Google Scholar]
- Ponte P., Gonzalez-DeWhitt P., Schilling J., Miller J., Hsu D., Greenberg B., Davis K., Wallace W., Lieberburg I., Fuller F. A new A4 amyloid mRNA contains a domain homologous to serine proteinase inhibitors. Nature. 1988 Feb 11;331(6156):525–527. doi: 10.1038/331525a0. [DOI] [PubMed] [Google Scholar]
- Probst A., Brunnschweiler H., Lautenschlager C., Ulrich J. A special type of senile plaque, possibly an initial stage. Acta Neuropathol. 1987;74(2):133–141. doi: 10.1007/BF00692843. [DOI] [PubMed] [Google Scholar]
- Quon D., Wang Y., Catalano R., Scardina J. M., Murakami K., Cordell B. Formation of beta-amyloid protein deposits in brains of transgenic mice. Nature. 1991 Jul 18;352(6332):239–241. doi: 10.1038/352239a0. [DOI] [PubMed] [Google Scholar]
- Refolo L. M., Salton S. R., Anderson J. P., Mehta P., Robakis N. K. Nerve and epidermal growth factors induce the release of the Alzheimer amyloid precursor from PC 12 cell cultures. Biochem Biophys Res Commun. 1989 Oct 31;164(2):664–670. doi: 10.1016/0006-291x(89)91511-8. [DOI] [PubMed] [Google Scholar]
- Robakis N. K., Ramakrishna N., Wolfe G., Wisniewski H. M. Molecular cloning and characterization of a cDNA encoding the cerebrovascular and the neuritic plaque amyloid peptides. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4190–4194. doi: 10.1073/pnas.84.12.4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozemuller J. M., Eikelenboom P., Stam F. C., Beyreuther K., Masters C. L. A4 protein in Alzheimer's disease: primary and secondary cellular events in extracellular amyloid deposition. J Neuropathol Exp Neurol. 1989 Nov;48(6):674–691. doi: 10.1097/00005072-198911000-00009. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J. Biochemistry of altered brain proteins in Alzheimer's disease. Annu Rev Neurosci. 1989;12:463–490. doi: 10.1146/annurev.ne.12.030189.002335. [DOI] [PubMed] [Google Scholar]
- Snow A. D., Kisilevsky R., Willmer J., Prusiner S. B., DeArmond S. J. Sulfated glycosaminoglycans in amyloid plaques of prion diseases. Acta Neuropathol. 1989;77(4):337–342. doi: 10.1007/BF00687367. [DOI] [PubMed] [Google Scholar]
- Stern R. A., Otvos L., Jr, Trojanowski J. Q., Lee V. M. Monoclonal antibodies to a synthetic peptide homologous with the first 28 amino acids of Alzheimer's disease beta-protein recognize amyloid and diverse glial and neuronal cell types in the central nervous system. Am J Pathol. 1989 May;134(5):973–978. [PMC free article] [PubMed] [Google Scholar]
- TERRY R. D., GONATAS N. K., WEISS M. ULTRASTRUCTURAL STUDIES IN ALZHEIMER'S PRESENILE DEMENTIA. Am J Pathol. 1964 Feb;44:269–297. [PMC free article] [PubMed] [Google Scholar]
- Tagliavini F., Ghiso J., Timmers W. F., Giaccone G., Bugiani O., Frangione B. Coexistence of Alzheimer's amyloid precursor protein and amyloid protein in cerebral vessel walls. Lab Invest. 1990 Jun;62(6):761–767. [PubMed] [Google Scholar]
- Tagliavini F., Giaccone G., Frangione B., Bugiani O. Preamyloid deposits in the cerebral cortex of patients with Alzheimer's disease and nondemented individuals. Neurosci Lett. 1988 Nov 11;93(2-3):191–196. doi: 10.1016/0304-3940(88)90080-8. [DOI] [PubMed] [Google Scholar]
- Takahashi H., Kurashima C., Utsuyama M., Hirokawa K. Immunohistological study of senile brains by using a monoclonal antibody recognizing beta amyloid precursor protein: significance of granular deposits in relation with senile plaques. Acta Neuropathol. 1990;80(3):260–265. doi: 10.1007/BF00294643. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Nakamura S., Ueda K., Kameyama M., Shiojiri S., Takahashi Y., Kitaguchi N., Ito H. Three types of amyloid protein precursor mRNA in human brain: their differential expression in Alzheimer's disease. Biochem Biophys Res Commun. 1988 Dec 15;157(2):472–479. doi: 10.1016/s0006-291x(88)80273-0. [DOI] [PubMed] [Google Scholar]
- Tanzi R. E., Gusella J. F., Watkins P. C., Bruns G. A., St George-Hyslop P., Van Keuren M. L., Patterson D., Pagan S., Kurnit D. M., Neve R. L. Amyloid beta protein gene: cDNA, mRNA distribution, and genetic linkage near the Alzheimer locus. Science. 1987 Feb 20;235(4791):880–884. doi: 10.1126/science.2949367. [DOI] [PubMed] [Google Scholar]
- Tanzi R. E., McClatchey A. I., Lamperti E. D., Villa-Komaroff L., Gusella J. F., Neve R. L. Protease inhibitor domain encoded by an amyloid protein precursor mRNA associated with Alzheimer's disease. Nature. 1988 Feb 11;331(6156):528–530. doi: 10.1038/331528a0. [DOI] [PubMed] [Google Scholar]
- Ulrich J. Alzheimer changes in nondemented patients younger than sixty-five: possible early stages of Alzheimer's disease and senile dementia of Alzheimer type. Ann Neurol. 1985 Mar;17(3):273–277. doi: 10.1002/ana.410170309. [DOI] [PubMed] [Google Scholar]
- Verga L., Frangione B., Tagliavini F., Giaccone G., Migheli A., Bugiani O. Alzheimer patients and Down patients: cerebral preamyloid deposits differ ultrastructurally and histochemically from the amyloid of senile plaques. Neurosci Lett. 1989 Nov 6;105(3):294–299. doi: 10.1016/0304-3940(89)90636-8. [DOI] [PubMed] [Google Scholar]
- Vinters H. V., Pardridge W. M., Secor D. L., Ishii N. Immunohistochemical study of cerebral amyloid angiopathy. II. Enhancement of immunostaining using formic acid pretreatment of tissue sections. Am J Pathol. 1988 Oct;133(1):150–162. [PMC free article] [PubMed] [Google Scholar]
- Vinters H. V., Pardridge W. M., Yang J. Immunohistochemical study of cerebral amyloid angiopathy: use of an antiserum to a synthetic 28-amino-acid peptide fragment of the Alzheimer's disease amyloid precursor. Hum Pathol. 1988 Feb;19(2):214–222. doi: 10.1016/s0046-8177(88)80352-6. [DOI] [PubMed] [Google Scholar]
- Wisniewski K. E., Maslinska D., Kitaguchi T., Kim K. S., Goebel H. H., Haltia M. Topographic heterogeneity of amyloid B-protein epitopes in brains with various forms of neuronal ceroid lipofuscinoses suggesting defective processing of amyloid precursor protein. Acta Neuropathol. 1990;80(1):26–34. doi: 10.1007/BF00294218. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H., Hirai S., Morimatsu M., Shoji M., Harigaya Y. Diffuse type of senile plaques in the brains of Alzheimer-type dementia. Acta Neuropathol. 1988;77(2):113–119. doi: 10.1007/BF00687420. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H., Hirai S., Morimatsu M., Shoji M., Ihara Y. A variety of cerebral amyloid deposits in the brains of the Alzheimer-type dementia demonstrated by beta protein immunostaining. Acta Neuropathol. 1988;76(6):541–549. doi: 10.1007/BF00689591. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H., Nakazato Y., Hirai S., Shoji M., Harigaya Y. Electron micrograph of diffuse plaques. Initial stage of senile plaque formation in the Alzheimer brain. Am J Pathol. 1989 Oct;135(4):593–597. [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Hirano A. A comparative study of modified Bielschowsky, Bodian and thioflavin S stains on Alzheimer's neurofibrillary tangles. Neuropathol Appl Neurobiol. 1986 Jan-Feb;12(1):3–9. doi: 10.1111/j.1365-2990.1986.tb00677.x. [DOI] [PubMed] [Google Scholar]
- de Sauvage F., Octave J. N. A novel mRNA of the A4 amyloid precursor gene coding for a possibly secreted protein. Science. 1989 Aug 11;245(4918):651–653. doi: 10.1126/science.2569763. [DOI] [PubMed] [Google Scholar]