Abstract
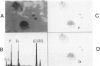
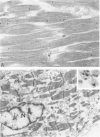
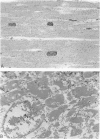
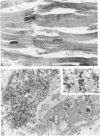
Low dietary Mg results in Ca loading of cardiac myocytes, which increases the likelihood of myocyte calcification in the event of acute myocardial infarction (AMI), and possibly increases myocyte vulnerability to necrosis. Bloom and Peric-Golia1 previously reported an autopsy study of cases from the Washington, D.C. area (a region with low levels of Mg in the drinking water), demonstrating AMI-associated mineralization in myocytes with histologically normal nuclei and cross striations, as well as in obviously necrotic myocytes. The authors have re-examined mineralized myocytes from the same autopsy material, using electron probe microanalysis, light microscopy, and transmission electron microscopy. Microprobe analysis identified Ca and P as the nuclides composing the inorganic phase of the mineral deposits. Ultrastructurally, all Ca deposits, regardless of size or intracellular location, were composed of aggregates of needlelike hydroxyapatite crystals. The mildest form of intracellular Ca deposition was observed as small Ca deposits limited to some mitochondria of myocytes, which demonstrated intact nuclei and regular sarcomere pattern. More advanced stages of intracellular calcification, in the form of Ca deposits associated with mitochondria, Z-band regions and nuclei, were observed in other myocytes that also retained intact nuclei and sarcomeres. Massive Ca deposits were associated with myocytes which showed morphologic features of advanced necrosis, including loss of nuclei, disruption of sarcomere structure and masses of cellular debris. These observations support the theory originally proposed by Bloom and Peric-Golia1 suggesting that Ca loading of myocytes, possibly related to Mg deficiency in humans, increased vulnerability of the myocytes to subsequent AMI-associated necrosis and dystrophic calcification. In addition, the light microscopic impression of calcification of otherwise normal myocytes is contradicted by the electron microscopic identification of hydroxyapatite crystals free in the sarcoplasm, a condition unlikely to be compatible with viability. Lastly, the fact that all Ca deposits were in the form of hydroxyapatite supports the view that they were formed in a Mg-poor environment, which favors conversion of the more common amorphous form of Ca phosphate into the needlelike crystals of hydroxyapatite.
Full text
PDF

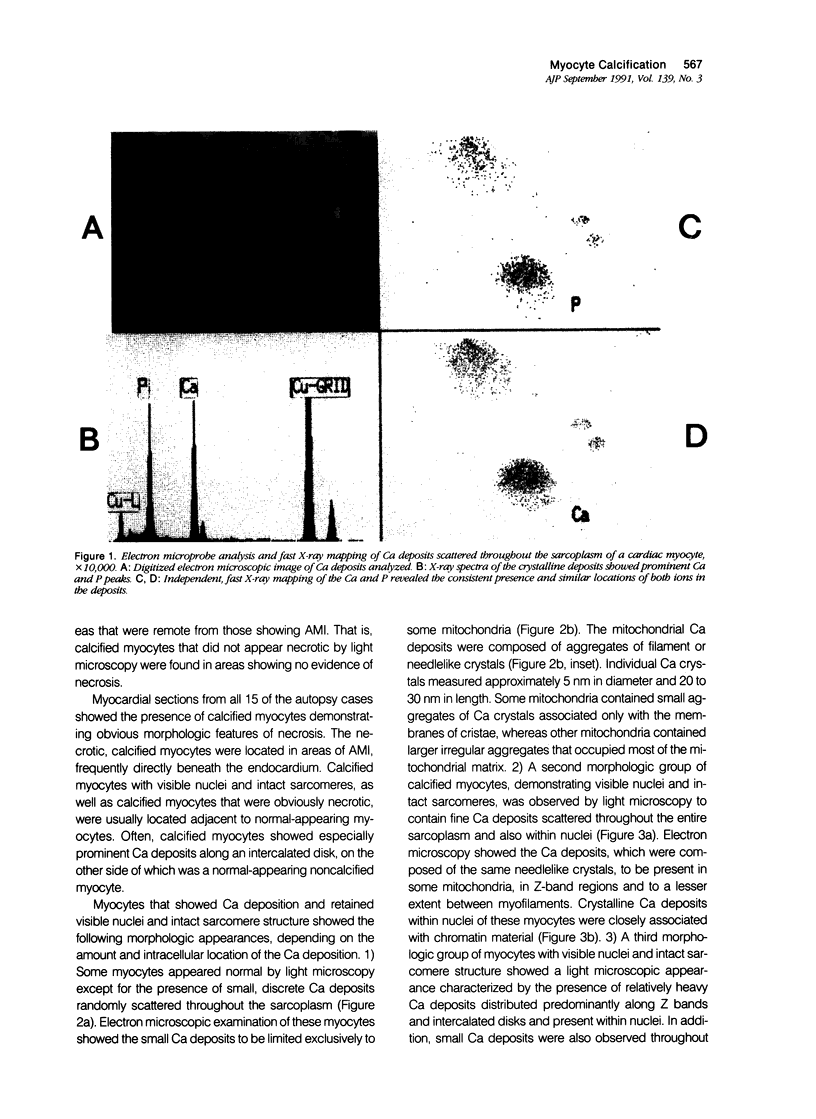

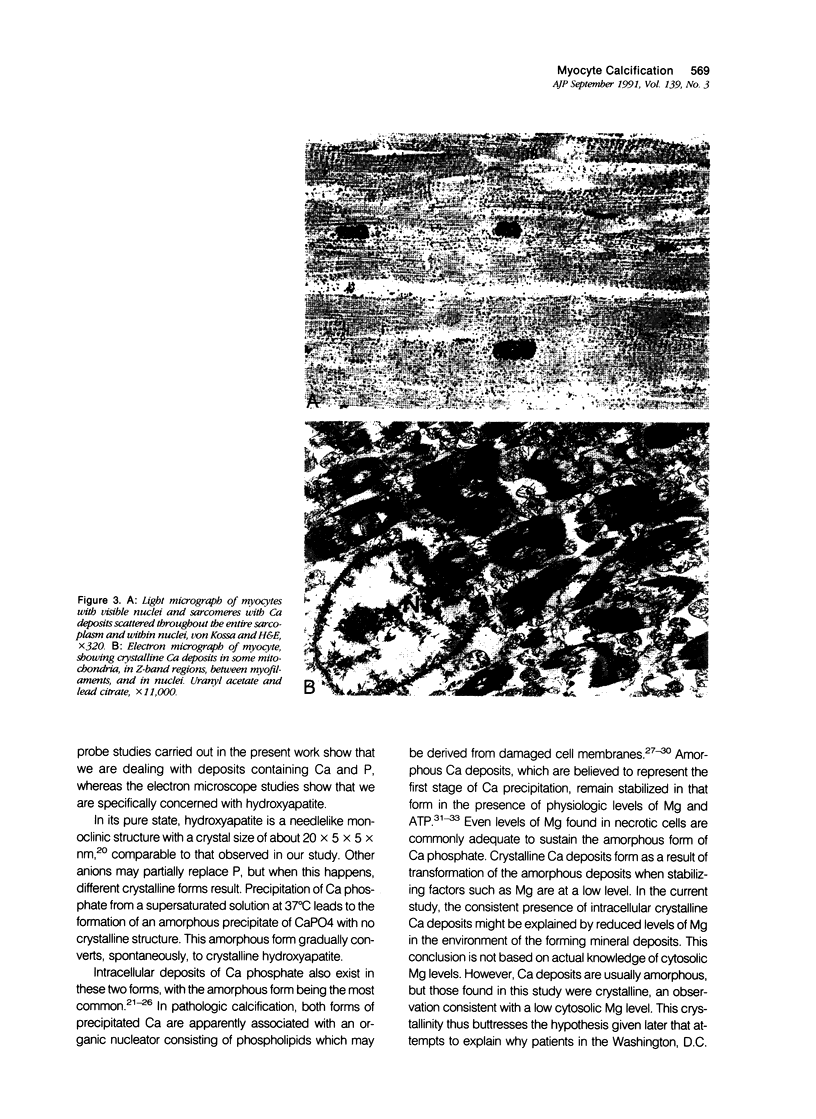
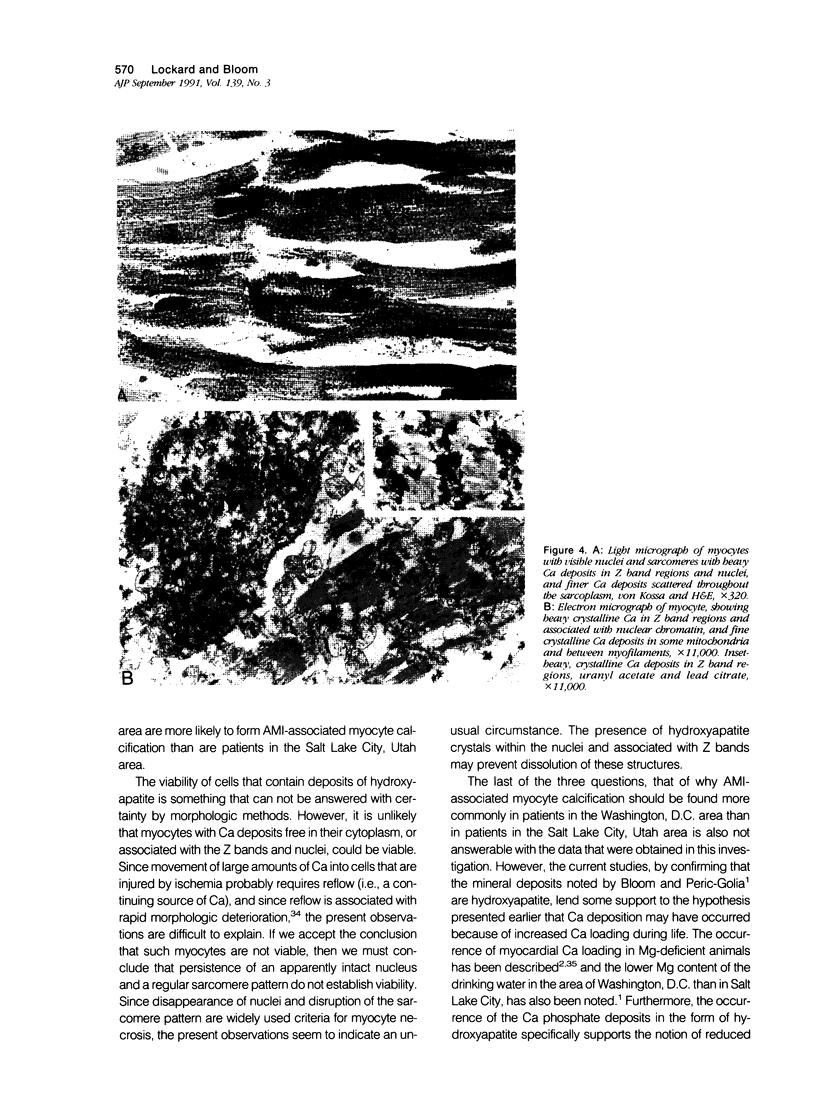


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson H. C. Calcific diseases. A concept. Arch Pathol Lab Med. 1983 Jul;107(7):341–348. [PubMed] [Google Scholar]
- Ashraf M., White F., Bloor C. M. Ultrastructural influence of reperfusing dog myocardium with calcium-free blood after coronary artery occlusion. Am J Pathol. 1978 Feb;90(2):423–434. [PMC free article] [PubMed] [Google Scholar]
- Bloom S. Magnesium deficiency cardiomyopathy. Am J Cardiovasc Pathol. 1988;2(1):7–17. [PubMed] [Google Scholar]
- Bloom S., Peric-Golia L. Geographic variation in the incidence of myocardial calcification associated with acute myocardial infarction. Hum Pathol. 1989 Aug;20(8):726–731. doi: 10.1016/0046-8177(89)90064-6. [DOI] [PubMed] [Google Scholar]
- Blumenthal N. C., Betts F., Posner A. S. Stabilization of amorphous calcium phosphate by Mg and ATP. Calcif Tissue Res. 1977 Oct 20;23(3):245–250. doi: 10.1007/BF02012793. [DOI] [PubMed] [Google Scholar]
- Bonucci E., Derenzini M., Marinozzi V. The organic-inorganic relationship in calcified mitochondria. J Cell Biol. 1973 Oct;59(1):185–211. doi: 10.1083/jcb.59.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonucci E., Sadun R. Experimental calcification of the myocardium. Ultrastructural and histochemical investigations. Am J Pathol. 1973 May;71(2):167–192. [PMC free article] [PubMed] [Google Scholar]
- Bonucci E., Silvestrini G., Di Grezia R. The ultrastructure of the organic phase associated with the inorganic substance in calcified tissues. Clin Orthop Relat Res. 1988 Aug;(233):243–261. [PubMed] [Google Scholar]
- Boskey A. L., Bullough P. G., Vigorita V., Di Carlo E. Calcium-acidic phospholipid-phosphate complexes in human hydroxyapatite-containing pathologic deposits. Am J Pathol. 1988 Oct;133(1):22–29. [PMC free article] [PubMed] [Google Scholar]
- CAULFIELD J. B., SCHRAG P. E. ELECTRON MICROSCOPIC STUDY OF RENAL CALCIFICATION. Am J Pathol. 1964 Mar;44:365–381. [PMC free article] [PubMed] [Google Scholar]
- Chang C., Bloom S. Interrelationship of dietary Mg intake and electrolyte homeostasis in hamsters: I. Severe Mg deficiency, electrolyte homeostasis, and myocardial necrosis. J Am Coll Nutr. 1985;4(2):173–185. doi: 10.1080/07315724.1985.10720074. [DOI] [PubMed] [Google Scholar]
- Chang C., Varghese P. J., Downey J., Bloom S. Magnesium deficiency and myocardial infarct size in the dog. J Am Coll Cardiol. 1985 Feb;5(2 Pt 1):280–289. doi: 10.1016/s0735-1097(85)80048-6. [DOI] [PubMed] [Google Scholar]
- D'Agostino A. N., Chiga M. Mitochondrial mineralization in human myocardium. Am J Clin Pathol. 1970 Jun;53(6):820–824. doi: 10.1093/ajcp/53.6.820. [DOI] [PubMed] [Google Scholar]
- Davis W. L., Jones R. G., Hagler H. K. An electron microscopic histochemical and analytical X-ray microprobe study of calcification in Bruch's membrane from human eyes. J Histochem Cytochem. 1981 May;29(5):601–608. doi: 10.1177/29.5.7252127. [DOI] [PubMed] [Google Scholar]
- Dyckner T. Serum magnesium in acute myocardial infarction. Relation to arrhythmias. Acta Med Scand. 1980;207(1-2):59–66. doi: 10.1111/j.0954-6820.1980.tb09676.x. [DOI] [PubMed] [Google Scholar]
- Ghidoni J. J., Liotta D., Thomas H. Massive subendocardial damage accompanying prolonged ventricular fibrillation. Am J Pathol. 1969 Jul;56(1):15–29. [PMC free article] [PubMed] [Google Scholar]
- HEGGTVEIT H. A., HERMAN L., MISHRA R. K. CARDIAC NECROSIS AND CALCIFICATION IN EXPERIMENTAL MAGNESIUM DEFICIENCY. A LIGHT AND ELECTRON MICROSCOPIC STUDY. Am J Pathol. 1964 Nov;45:757–782. [PMC free article] [PubMed] [Google Scholar]
- Johnson C. J., Peterson D. R., Smith E. K. Myocardial tissue concentrations of magnesium and potassium in men dying suddenly from ischemic heart disease. Am J Clin Nutr. 1979 May;32(5):967–970. doi: 10.1093/ajcn/32.5.967. [DOI] [PubMed] [Google Scholar]
- Karppanen H., Pennanen R., Passinen L. Minerals, coronary heart disease and sudden coronary death. Adv Cardiol. 1978;25:9–24. doi: 10.1159/000402000. [DOI] [PubMed] [Google Scholar]
- Leary W. P. Content of magnesium in drinking water and deaths from ischaemic heart disease in white South Africans. Magnesium. 1986;5(3-4):150–153. [PubMed] [Google Scholar]
- Legato M. J., Spiro D., Langer G. A. Ultrastructural alterations produced in mammalian myocardium by variation in perfusate ionic composition. J Cell Biol. 1968 Apr;37(1):1–12. doi: 10.1083/jcb.37.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. J. Intramitochondrial calcification in infant myocardium. Occurrence in a case of coarctation of aorta. Arch Pathol. 1972 Oct;94(4):366–369. [PubMed] [Google Scholar]
- Marier J. R., Neri L. C. Quantifying the role of magnesium in the interrelationship between human mortality/morbidity and water hardness. Magnesium. 1985;4(2-3):53–59. [PubMed] [Google Scholar]
- Morgan K. J., Stampley G. L., Zabik M. E., Fischer D. R. Magnesium and calcium dietary intakes of the U.S. population. J Am Coll Nutr. 1985;4(2):195–206. doi: 10.1080/07315724.1985.10720076. [DOI] [PubMed] [Google Scholar]
- Neri L. C., Johansen H. L. Water hardness and cardiovascular mortality. Ann N Y Acad Sci. 1978 Mar 30;304:203–221. doi: 10.1111/j.1749-6632.1978.tb25595.x. [DOI] [PubMed] [Google Scholar]
- Posner A. S. Intramitochondrial storage of stable amorphous calcium phosphate. Ann N Y Acad Sci. 1978 Apr 28;307:248–249. doi: 10.1111/j.1749-6632.1978.tb41955.x. [DOI] [PubMed] [Google Scholar]
- Punsar S., Karvonen M. J. Drinking water quality and sudden death: observations from West and East Finland. Cardiology. 1979;64(1):24–34. doi: 10.1159/000170575. [DOI] [PubMed] [Google Scholar]
- Trump B. F., Berezesky I. K., Laiho K. U., Osornio A. R., Mergner W. J., Smith M. W. The role of calcium in cell injury. A review. Scan Electron Microsc. 1980;(Pt 2):437-62, 492. [PubMed] [Google Scholar]
- Trump B. F., Berezesky I. K. The role of calcium in cell injury and repair: a hypothesis. Surv Synth Pathol Res. 1985;4(3):248–256. doi: 10.1159/000156978. [DOI] [PubMed] [Google Scholar]
- Wrogemann K., Pena S. D. Mitochondrial calcium overload: A general mechanism for cell-necrosis in muscle diseases. Lancet. 1976 Mar 27;1(7961):672–674. doi: 10.1016/s0140-6736(76)92781-1. [DOI] [PubMed] [Google Scholar]