Abstract
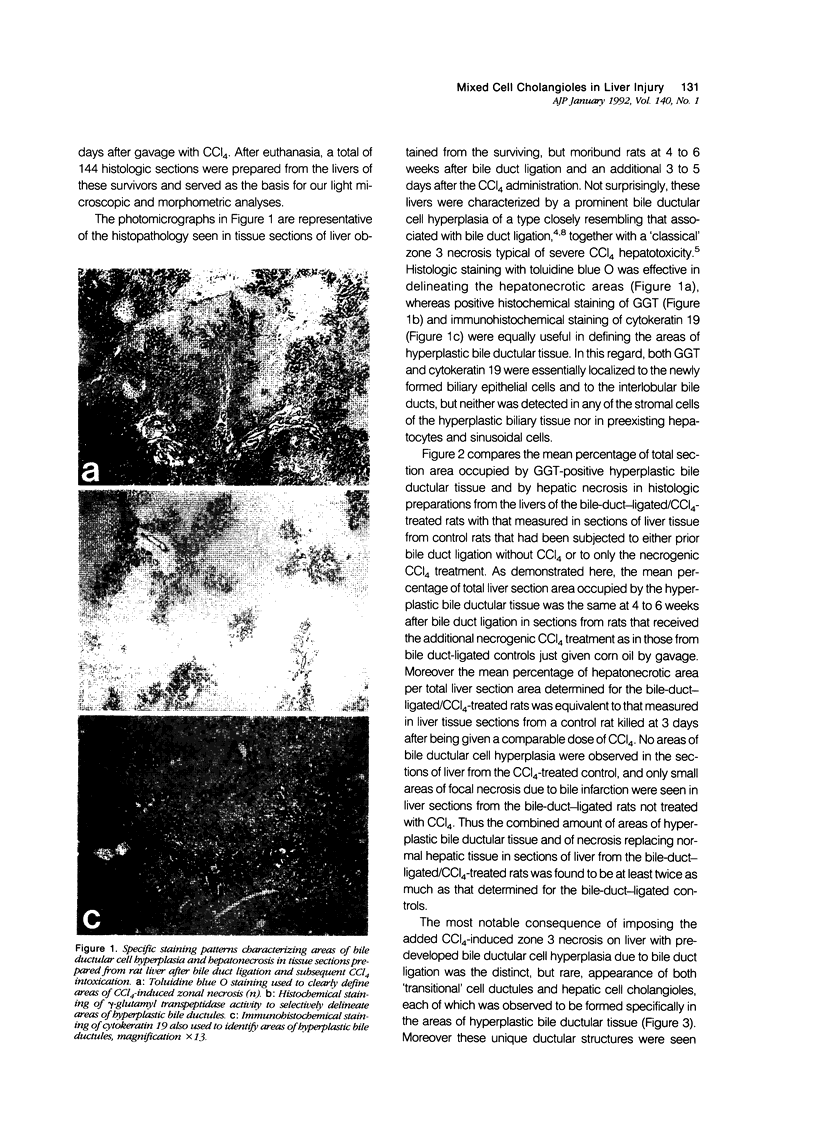
Intrahepatic biliary cell plasticity was investigated in a rat model that combined prior bile ductular cell hyperplasia after bile duct ligation with subsequent CCl4-induced hepatonecrosis. Morphometric analysis of histologic liver sections from rats at 4 to 6 weeks after bile duct ligation and 3 to 5 days after CCl4 demonstrated the total section area to be occupied by near-equal amounts of hyperplastic bile ductular tissue area and hepatonecrotic area. Of particular significance was the unique presence, albeit infrequent, of newly appearing hepatic cell cholangioles composed of both biliary epithelial cells and one or more 'ductular hepatocytes' exclusively within the hyperplastic bile ductular tissue area of liver sections from the bile-duct-ligated/CCl4-treated rats, but not observed in control liver sections. This finding is compatible with the possibility of a 'transdifferentiation' of some hyperplastic biliary epithelial cells into 'ductular hepatocytes' in response to an extreme hepatic injury.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpini G., Lenzi R., Zhai W. R., Slott P. A., Liu M. H., Sarkozi L., Tavoloni N. Bile secretory function of intrahepatic biliary epithelium in the rat. Am J Physiol. 1989 Jul;257(1 Pt 1):G124–G133. doi: 10.1152/ajpgi.1989.257.1.G124. [DOI] [PubMed] [Google Scholar]
- Desmet V. J. Current problems in diagnosis of biliary disease and cholestasis. Semin Liver Dis. 1986 Aug;6(3):233–245. doi: 10.1055/s-2008-1040606. [DOI] [PubMed] [Google Scholar]
- Desmet V. J. Intrahepatic bile ducts under the lens. J Hepatol. 1985;1(5):545–559. doi: 10.1016/s0168-8278(85)80752-2. [DOI] [PubMed] [Google Scholar]
- Dunsford H. A., Maset R., Salman J., Sell S. Connection of ductlike structures induced by a chemical hepatocarcinogen to portal bile ducts in the rat liver detected by injection of bile ducts with a pigmented barium gelatin medium. Am J Pathol. 1985 Feb;118(2):218–224. [PMC free article] [PubMed] [Google Scholar]
- Elmore L. W., Sirica A. E. Phenotypic characterization of metaplastic intestinal glands and ductular hepatocytes in cholangiofibrotic lesions rapidly induced in the caudate liver lobe of rats treated with furan. Cancer Res. 1991 Oct 15;51(20):5752–5759. [PubMed] [Google Scholar]
- Evarts R. P., Nagy P., Marsden E., Thorgeirsson S. S. A precursor-product relationship exists between oval cells and hepatocytes in rat liver. Carcinogenesis. 1987 Nov;8(11):1737–1740. doi: 10.1093/carcin/8.11.1737. [DOI] [PubMed] [Google Scholar]
- Evarts R. P., Nagy P., Nakatsukasa H., Marsden E., Thorgeirsson S. S. In vivo differentiation of rat liver oval cells into hepatocytes. Cancer Res. 1989 Mar 15;49(6):1541–1547. [PubMed] [Google Scholar]
- Faris R. A., Monfils B. A., Dunsford H. A., Hixson D. C. Antigenic relationship between oval cells and a subpopulation of hepatic foci, nodules, and carcinomas induced by the "resistant hepatocyte" model system. Cancer Res. 1991 Feb 15;51(4):1308–1317. [PubMed] [Google Scholar]
- Fausto N. Hepatocyte differentiation and liver progenitor cells. Curr Opin Cell Biol. 1990 Dec;2(6):1036–1042. doi: 10.1016/0955-0674(90)90153-6. [DOI] [PubMed] [Google Scholar]
- Gerber M. A., Thung S. N., Shen S., Stromeyer F. W., Ishak K. G. Phenotypic characterization of hepatic proliferation. Antigenic expression by proliferating epithelial cells in fetal liver, massive hepatic necrosis, and nodular transformation of the liver. Am J Pathol. 1983 Jan;110(1):70–74. [PMC free article] [PubMed] [Google Scholar]
- Grisham J. W., Tsao M. S., Lee D. C., Earp H. S. Sequential changes in epidermal growth factor receptor/ligand function in cultured rat liver epithelial cells transformed chemically in vitro. Pathobiology. 1990;58(1):3–14. doi: 10.1159/000163560. [DOI] [PubMed] [Google Scholar]
- Harris L., Morris L. E., Farber E. Protective value of a liver initiation-promotion regimen against the lethal effect of carbon tetrachloride in rats. Lab Invest. 1989 Oct;61(4):467–470. [PubMed] [Google Scholar]
- Hixson D. C., Faris R. A., Thompson N. L. An antigenic portrait of the liver during carcinogenesis. Pathobiology. 1990;58(2):65–77. doi: 10.1159/000163565. [DOI] [PubMed] [Google Scholar]
- Lemire J. M., Shiojiri N., Fausto N. Oval cell proliferation and the origin of small hepatocytes in liver injury induced by D-galactosamine. Am J Pathol. 1991 Sep;139(3):535–552. [PMC free article] [PubMed] [Google Scholar]
- Makino T., Usuda N., Rao S., Reddy J. K., Scarpelli D. G. Transdifferentiation of ductular cells into hepatocytes in regenerating hamster pancreas. Lab Invest. 1990 May;62(5):552–561. [PubMed] [Google Scholar]
- Marceau N. Cell lineages and differentiation programs in epidermal, urothelial and hepatic tissues and their neoplasms. Lab Invest. 1990 Jul;63(1):4–20. [PubMed] [Google Scholar]
- Mathis G. A., Walls S. A., D'Amico P., Gengo T. F., Sirica A. E. Enzyme profile of rat bile ductular epithelial cells in reference to the resistance phenotype in hepatocarcinogenesis. Hepatology. 1989 Mar;9(3):477–485. doi: 10.1002/hep.1840090323. [DOI] [PubMed] [Google Scholar]
- Mathis G. A., Walls S. A., Sirica A. E. Biochemical characteristics of hyperplastic rat bile ductular epithelial cells cultured "on top" and "inside" different extracellular matrix substitutes. Cancer Res. 1988 Nov 1;48(21):6145–6153. [PubMed] [Google Scholar]
- Petropoulos C. J., Yaswen P., Panzica M., Fausto N. Cell lineages in liver carcinogenesis: possible clues from studies of the distribution of alpha-fetoprotein RNA sequences in cell populations isolated from normal, regenerating, and preneoplastic rat livers. Cancer Res. 1985 Nov;45(11 Pt 2):5762–5768. [PubMed] [Google Scholar]
- Phillips M. J., Poucell S. Modern aspects of the morphology of viral hepatitis. Hum Pathol. 1981 Dec;12(12):1060–1084. doi: 10.1016/s0046-8177(81)80328-0. [DOI] [PubMed] [Google Scholar]
- Rao M. S., Dwivedi R. S., Yeldandi A. V., Subbarao V., Tan X. D., Usman M. I., Thangada S., Nemali M. R., Kumar S., Scarpelli D. G. Role of periductal and ductular epithelial cells of the adult rat pancreas in pancreatic hepatocyte lineage. A change in the differentiation commitment. Am J Pathol. 1989 May;134(5):1069–1086. [PMC free article] [PubMed] [Google Scholar]
- Rao M. S., Yeldandi A. V., Reddy J. K. Differentiation and cell proliferation patterns in rat exocrine pancreas: role of type I and type II injury. Pathobiology. 1990;58(1):37–43. doi: 10.1159/000163563. [DOI] [PubMed] [Google Scholar]
- Rutenburg A. M., Kim H., Fischbein J. W., Hanker J. S., Wasserkrug H. L., Seligman A. M. Histochemical and ultrastructural demonstration of gamma-glutamyl transpeptidase activity. J Histochem Cytochem. 1969 Aug;17(8):517–526. doi: 10.1177/17.8.517. [DOI] [PubMed] [Google Scholar]
- Sell S. Comparison of oval cells induced in rat liver by feeding N-2-fluorenylacetamide in a choline-devoid diet and bile duct cells induced by feeding 4,4'-diaminodiphenylmethane. Cancer Res. 1983 Apr;43(4):1761–1767. [PubMed] [Google Scholar]
- Sell S. Distribution of alpha-fetoprotein- and albumin-containing cells in the livers of Fischer rats fed four cycles of N-2-fluorenylacetamide. Cancer Res. 1978 Sep;38(9):3107–3113. [PubMed] [Google Scholar]
- Sell S., Salman J. Light- and electron-microscopic autoradiographic analysis of proliferating cells during the early stages of chemical hepatocarcinogenesis in the rat induced by feeding N-2-fluorenylacetamide in a choline-deficient diet. Am J Pathol. 1984 Feb;114(2):287–300. [PMC free article] [PubMed] [Google Scholar]
- Sirica A. E., Cihla H. P. Isolation and partial characterizations of oval and hyperplastic bile ductular cell-enriched populations from the livers of carcinogen and noncarcinogen-treated rats. Cancer Res. 1984 Aug;44(8):3454–3466. [PubMed] [Google Scholar]
- Sirica A. E., Mathis G. A., Sano N., Elmore L. W. Isolation, culture, and transplantation of intrahepatic biliary epithelial cells and oval cells. Pathobiology. 1990;58(1):44–64. doi: 10.1159/000163564. [DOI] [PubMed] [Google Scholar]
- Sirica A. E., Wilkerson C. S., Wu L. L., Fitzgerald R., Blanke R. V., Guzelian P. S. Evaluation of chlordecone in a two-stage model of hepatocarcinogenesis: a significant sex difference in the hepatocellular carcinoma incidence. Carcinogenesis. 1989 Jun;10(6):1047–1054. doi: 10.1093/carcin/10.6.1047. [DOI] [PubMed] [Google Scholar]
- Tournier I., Legrès L., Schoevaert D., Feldmann G., Bernuau D. Cellular analysis of alpha-fetoprotein gene activation during carbon tetrachloride and D-galactosamine-induced acute liver injury in rats. Lab Invest. 1988 Nov;59(5):657–665. [PubMed] [Google Scholar]
- Vandersteenhoven A. M., Burchette J., Michalopoulos G. Characterization of ductular hepatocytes in end-stage cirrhosis. Arch Pathol Lab Med. 1990 Apr;114(4):403–406. [PubMed] [Google Scholar]
- Wu P. C., Ma L., Gibson J. B., Hirai H., Tsukada Y. Serum alpha-fetoprotein in rats after ligation of the common bile duct: relation to ductular cell (oval cell) proliferation. J Pathol. 1981 Jan;133(1):61–74. doi: 10.1002/path.1711330107. [DOI] [PubMed] [Google Scholar]




