Abstract
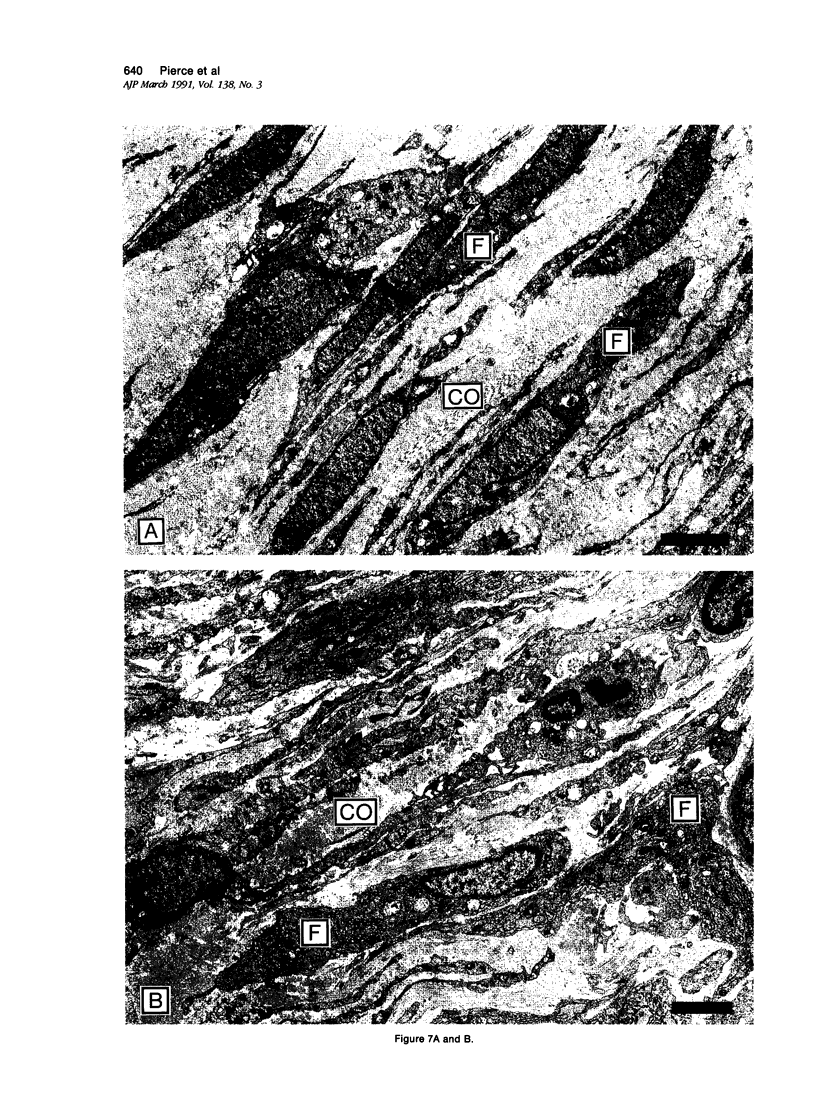
Recombinant platelet-derived growth factor (PDGF) and transforming growth factor beta 1 (TGF-beta 1) influence the rate of extracellular matrix formed in treated incisional wounds. Because incisional healing processes are difficult to quantify, a full-thickness excisional wound model in the rabbit ear was developed to permit detailed analyses of growth-factor-mediated tissue repair. In the present studies, quantitative and qualitative differences in acute inflammatory cell influx, glycosaminoglycan (GAG) deposition, collagen formation, and myofibroblast generation in PDGF-BB (BB homodimer)- and TGF-beta 1-treated wounds were detected when analyzed histochemically and ultrastructurally. Although both growth factors significantly augmented extracellular matrix formation and healing in 10-day wounds compared with controls (P less than 0.002). PDGF-BB markedly increased macrophage influx and GAG deposition, whereas TGF-beta 1 selectively induced significantly more mature collagen bundles at the leading edge of new granulation tissue (P = 0.007). Transforming growth factor-beta 1-treated wound fibroblasts demonstrated active collagen fibrillogenesis and accretion of subfibrils at the ultrastructural level. Myofibroblasts, phenotypically modified fibroblasts considered responsible for wound contraction, were observed in control, but were absent in early growth-factor-treated granulating wounds. These results provide important insights into the mechanisms of soft tissue repair and indicate that 1) PDGF-BB induces an inflammatory response and provisional matrix synthesis within wounds that is qualitatively similar but quantitatively increased compared with normal wounds; 2) TGF-beta 1 preferentially triggers synthesis and more rapid maturation of collagen within early wounds; and 3) both growth factors inhibit the differentiation of fibroblasts into myofibroblasts, perhaps because wound contraction is not required, due to increased extracellular matrix synthesis.
Full text
PDF
















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander S. A., Donoff R. B. The glycosaminoglycans of open wounds. J Surg Res. 1980 Nov;29(5):422–429. doi: 10.1016/0022-4804(80)90055-4. [DOI] [PubMed] [Google Scholar]
- Allen-Hoffmann B. L., Schlosser S. J., Brondyk W. H., Fahl W. E. Fibronectin levels are enhanced in human fibroblasts overexpressing the c-sis protooncogene. J Biol Chem. 1990 Mar 25;265(9):5219–5225. [PubMed] [Google Scholar]
- Assoian R. K., Fleurdelys B. E., Stevenson H. C., Miller P. J., Madtes D. K., Raines E. W., Ross R., Sporn M. B. Expression and secretion of type beta transforming growth factor by activated human macrophages. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6020–6024. doi: 10.1073/pnas.84.17.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassols A., Massagué J. Transforming growth factor beta regulates the expression and structure of extracellular matrix chondroitin/dermatan sulfate proteoglycans. J Biol Chem. 1988 Feb 25;263(6):3039–3045. [PubMed] [Google Scholar]
- Bauer E. A., Cooper T. W., Huang J. S., Altman J., Deuel T. F. Stimulation of in vitro human skin collagenase expression by platelet-derived growth factor. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4132–4136. doi: 10.1073/pnas.82.12.4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatti S. P., Foster D. N., Ranganathan G., Moses H. L., Getz M. J. Induction of fibronectin gene transcription and mRNA is a primary response to growth-factor stimulation of AKR-2B cells. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1119–1123. doi: 10.1073/pnas.85.4.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S. L., Rifas L., Shen V., Tong B., Pierce G., Deuel T., Peck W. A. J774A.1 macrophage cell line produces PDGF-like and non-PDGF-like growth factors for bone cells. J Bone Miner Res. 1987 Oct;2(5):467–474. doi: 10.1002/jbmr.5650020515. [DOI] [PubMed] [Google Scholar]
- Clark R. A., Folkvord J. M., Hart C. E., Murray M. J., McPherson J. M. Platelet isoforms of platelet-derived growth factor stimulate fibroblasts to contract collagen matrices. J Clin Invest. 1989 Sep;84(3):1036–1040. doi: 10.1172/JCI114227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUNPHY J. E., UDUPA K. N. Chemical and histochemical sequences in the normal healing of wounds. N Engl J Med. 1955 Nov 17;253(20):847–851. doi: 10.1056/NEJM195511172532002. [DOI] [PubMed] [Google Scholar]
- Deuel T. F. Polypeptide growth factors: roles in normal and abnormal cell growth. Annu Rev Cell Biol. 1987;3:443–492. doi: 10.1146/annurev.cb.03.110187.002303. [DOI] [PubMed] [Google Scholar]
- Deuel T. F., Senior R. M., Huang J. S., Griffin G. L. Chemotaxis of monocytes and neutrophils to platelet-derived growth factor. J Clin Invest. 1982 Apr;69(4):1046–1049. doi: 10.1172/JCI110509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy R. J., Petro J. A., Tomasek J. J. Evidence for the nonmuscle nature of the "myofibroblast" of granulation tissue and hypertropic scar. An immunofluorescence study. Am J Pathol. 1988 Feb;130(2):252–260. [PMC free article] [PubMed] [Google Scholar]
- Edwards D. R., Murphy G., Reynolds J. J., Whitham S. E., Docherty A. J., Angel P., Heath J. K. Transforming growth factor beta modulates the expression of collagenase and metalloproteinase inhibitor. EMBO J. 1987 Jul;6(7):1899–1904. doi: 10.1002/j.1460-2075.1987.tb02449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engrav L. H., Richey K. J., Kao C. C., Murray M. J. Topical growth factors and wound contraction in the rat: Part II. Platelet-derived growth factor and wound contraction in normal and steroid-impaired rats. Ann Plast Surg. 1989 Sep;23(3):245–248. doi: 10.1097/00000637-198909000-00009. [DOI] [PubMed] [Google Scholar]
- Florini J. R., Roberts A. B., Ewton D. Z., Falen S. L., Flanders K. C., Sporn M. B. Transforming growth factor-beta. A very potent inhibitor of myoblast differentiation, identical to the differentiation inhibitor secreted by Buffalo rat liver cells. J Biol Chem. 1986 Dec 15;261(35):16509–16513. [PubMed] [Google Scholar]
- Gabbiani G., Hirschel B. J., Ryan G. B., Statkov P. R., Majno G. Granulation tissue as a contractile organ. A study of structure and function. J Exp Med. 1972 Apr 1;135(4):719–734. doi: 10.1084/jem.135.4.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhalgh D. G., Sprugel K. H., Murray M. J., Ross R. PDGF and FGF stimulate wound healing in the genetically diabetic mouse. Am J Pathol. 1990 Jun;136(6):1235–1246. [PMC free article] [PubMed] [Google Scholar]
- Hay E. D. Extracellular matrix. J Cell Biol. 1981 Dec;91(3 Pt 2):205s–223s. doi: 10.1083/jcb.91.3.205s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin P., Laurent T. C., Heldin C. H. Effect of growth factors on hyaluronan synthesis in cultured human fibroblasts. Biochem J. 1989 Mar 15;258(3):919–922. doi: 10.1042/bj2580919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignotz R. A., Massagué J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986 Mar 25;261(9):4337–4345. [PubMed] [Google Scholar]
- Junqueira L. C., Montes G. S., Sanchez E. M. The influence of tissue section thickness on the study of collagen by the Picrosirius-polarization method. Histochemistry. 1982;74(1):153–156. doi: 10.1007/BF00495061. [DOI] [PubMed] [Google Scholar]
- Keski-Oja J., Raghow R., Sawdey M., Loskutoff D. J., Postlethwaite A. E., Kang A. H., Moses H. L. Regulation of mRNAs for type-1 plasminogen activator inhibitor, fibronectin, and type I procollagen by transforming growth factor-beta. Divergent responses in lung fibroblasts and carcinoma cells. J Biol Chem. 1988 Mar 5;263(7):3111–3115. [PubMed] [Google Scholar]
- Kurkinen M., Vaheri A., Roberts P. J., Stenman S. Sequential appearance of fibronectin and collagen in experimental granulation tissue. Lab Invest. 1980 Jul;43(1):47–51. [PubMed] [Google Scholar]
- Leibovich S. J., Ross R. The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum. Am J Pathol. 1975 Jan;78(1):71–100. [PMC free article] [PubMed] [Google Scholar]
- Leof E. B., Proper J. A., Goustin A. S., Shipley G. D., DiCorleto P. E., Moses H. L. Induction of c-sis mRNA and activity similar to platelet-derived growth factor by transforming growth factor beta: a proposed model for indirect mitogenesis involving autocrine activity. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2453–2457. doi: 10.1073/pnas.83.8.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J., Cheifetz S., Endo T., Nadal-Ginard B. Type beta transforming growth factor is an inhibitor of myogenic differentiation. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8206–8210. doi: 10.1073/pnas.83.21.8206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath M. H., Hundahl S. A. The spatial and temporal quantification of myofibroblasts. Plast Reconstr Surg. 1982 Jun;69(6):975–985. doi: 10.1097/00006534-198206000-00012. [DOI] [PubMed] [Google Scholar]
- Mustoe T. A., Purdy J., Gramates P., Deuel T. F., Thomason A., Pierce G. F. Reversal of impaired wound healing in irradiated rats by platelet-derived growth factor-BB. Am J Surg. 1989 Oct;158(4):345–350. doi: 10.1016/0002-9610(89)90131-1. [DOI] [PubMed] [Google Scholar]
- Oda D., Gown A. M., Vande Berg J. S., Stern R. The fibroblast-like nature of myofibroblasts. Exp Mol Pathol. 1988 Dec;49(3):316–329. doi: 10.1016/0014-4800(88)90004-4. [DOI] [PubMed] [Google Scholar]
- Overall C. M., Wrana J. L., Sodek J. Independent regulation of collagenase, 72-kDa progelatinase, and metalloendoproteinase inhibitor expression in human fibroblasts by transforming growth factor-beta. J Biol Chem. 1989 Jan 25;264(3):1860–1869. [PubMed] [Google Scholar]
- Paulsson Y., Hammacher A., Heldin C. H., Westermark B. Possible positive autocrine feedback in the prereplicative phase of human fibroblasts. Nature. 1987 Aug 20;328(6132):715–717. doi: 10.1038/328715a0. [DOI] [PubMed] [Google Scholar]
- Penttinen R. P., Kobayashi S., Bornstein P. Transforming growth factor beta increases mRNA for matrix proteins both in the presence and in the absence of changes in mRNA stability. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1105–1108. doi: 10.1073/pnas.85.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pick R., Jalil J. E., Janicki J. S., Weber K. T. The fibrillar nature and structure of isoproterenol-induced myocardial fibrosis in the rat. Am J Pathol. 1989 Feb;134(2):365–371. [PMC free article] [PubMed] [Google Scholar]
- Pierce G. F., Mustoe T. A., Lingelbach J., Masakowski V. R., Gramates P., Deuel T. F. Transforming growth factor beta reverses the glucocorticoid-induced wound-healing deficit in rats: possible regulation in macrophages by platelet-derived growth factor. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2229–2233. doi: 10.1073/pnas.86.7.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce G. F., Mustoe T. A., Lingelbach J., Masakowski V. R., Griffin G. L., Senior R. M., Deuel T. F. Platelet-derived growth factor and transforming growth factor-beta enhance tissue repair activities by unique mechanisms. J Cell Biol. 1989 Jul;109(1):429–440. doi: 10.1083/jcb.109.1.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce G. F., Mustoe T. A., Senior R. M., Reed J., Griffin G. L., Thomason A., Deuel T. F. In vivo incisional wound healing augmented by platelet-derived growth factor and recombinant c-sis gene homodimeric proteins. J Exp Med. 1988 Mar 1;167(3):974–987. doi: 10.1084/jem.167.3.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappolee D. A., Mark D., Banda M. J., Werb Z. Wound macrophages express TGF-alpha and other growth factors in vivo: analysis by mRNA phenotyping. Science. 1988 Aug 5;241(4866):708–712. doi: 10.1126/science.3041594. [DOI] [PubMed] [Google Scholar]
- Roberts C. J., Birkenmeier T. M., McQuillan J. J., Akiyama S. K., Yamada S. S., Chen W. T., Yamada K. M., McDonald J. A. Transforming growth factor beta stimulates the expression of fibronectin and of both subunits of the human fibronectin receptor by cultured human lung fibroblasts. J Biol Chem. 1988 Apr 5;263(10):4586–4592. [PubMed] [Google Scholar]
- Ross R., Benditt E. P. Wound healing and collagen formation. V. Quantitative electron microscope radioautographic observations of proline-H3 utilization by fibroblasts. J Cell Biol. 1965 Oct;27(1):83–106. doi: 10.1083/jcb.27.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R. The fibroblast and wound repair. Biol Rev Camb Philos Soc. 1968 Feb;43(1):51–96. doi: 10.1111/j.1469-185x.1968.tb01109.x. [DOI] [PubMed] [Google Scholar]
- Rossi P., Karsenty G., Roberts A. B., Roche N. S., Sporn M. B., de Crombrugghe B. A nuclear factor 1 binding site mediates the transcriptional activation of a type I collagen promoter by transforming growth factor-beta. Cell. 1988 Feb 12;52(3):405–414. doi: 10.1016/s0092-8674(88)80033-3. [DOI] [PubMed] [Google Scholar]
- Rudolph R., Guber S., Suzuki M., Woodward M. The life cycle of the myofibroblast. Surg Gynecol Obstet. 1977 Sep;145(3):389–394. [PubMed] [Google Scholar]
- Senior R. M., Griffin G. L., Huang J. S., Walz D. A., Deuel T. F. Chemotactic activity of platelet alpha granule proteins for fibroblasts. J Cell Biol. 1983 Feb;96(2):382–385. doi: 10.1083/jcb.96.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seppä H., Grotendorst G., Seppä S., Schiffmann E., Martin G. R. Platelet-derived growth factor in chemotactic for fibroblasts. J Cell Biol. 1982 Feb;92(2):584–588. doi: 10.1083/jcb.92.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalli O., Schürch W., Seemayer T., Lagacé R., Montandon D., Pittet B., Gabbiani G. Myofibroblasts from diverse pathologic settings are heterogeneous in their content of actin isoforms and intermediate filament proteins. Lab Invest. 1989 Feb;60(2):275–285. [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B., Wakefield L. M., de Crombrugghe B. Some recent advances in the chemistry and biology of transforming growth factor-beta. J Cell Biol. 1987 Sep;105(3):1039–1045. doi: 10.1083/jcb.105.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzeng D. Y., Deuel T. F., Huang J. S., Baehner R. L. Platelet-derived growth factor promotes human peripheral monocyte activation. Blood. 1985 Jul;66(1):179–183. [PubMed] [Google Scholar]
- Van Obberghen-Schilling E., Roche N. S., Flanders K. C., Sporn M. B., Roberts A. B. Transforming growth factor beta 1 positively regulates its own expression in normal and transformed cells. J Biol Chem. 1988 Jun 5;263(16):7741–7746. [PubMed] [Google Scholar]
- Vande Berg J. S., Rudolph R., Poolman W. L., Disharoon D. R. Comparative growth dynamics and actin concentration between cultured human myofibroblasts from granulating wounds and dermal fibroblasts from normal skin. Lab Invest. 1989 Nov;61(5):532–538. [PubMed] [Google Scholar]
- Vande Berg J. S., Rudolph R., Woodward M. Comparative growth dynamics and morphology between cultured myofibroblasts from granulating wounds and dermal fibroblasts. Am J Pathol. 1984 Feb;114(2):187–200. [PMC free article] [PubMed] [Google Scholar]
- Wahl S. M., Hunt D. A., Wakefield L. M., McCartney-Francis N., Wahl L. M., Roberts A. B., Sporn M. B. Transforming growth factor type beta induces monocyte chemotaxis and growth factor production. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5788–5792. doi: 10.1073/pnas.84.16.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch M. P., Odland G. F., Clark R. A. Temporal relationships of F-actin bundle formation, collagen and fibronectin matrix assembly, and fibronectin receptor expression to wound contraction. J Cell Biol. 1990 Jan;110(1):133–145. doi: 10.1083/jcb.110.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]












