Abstract
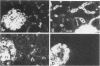
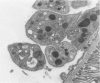
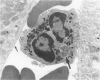
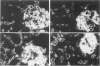
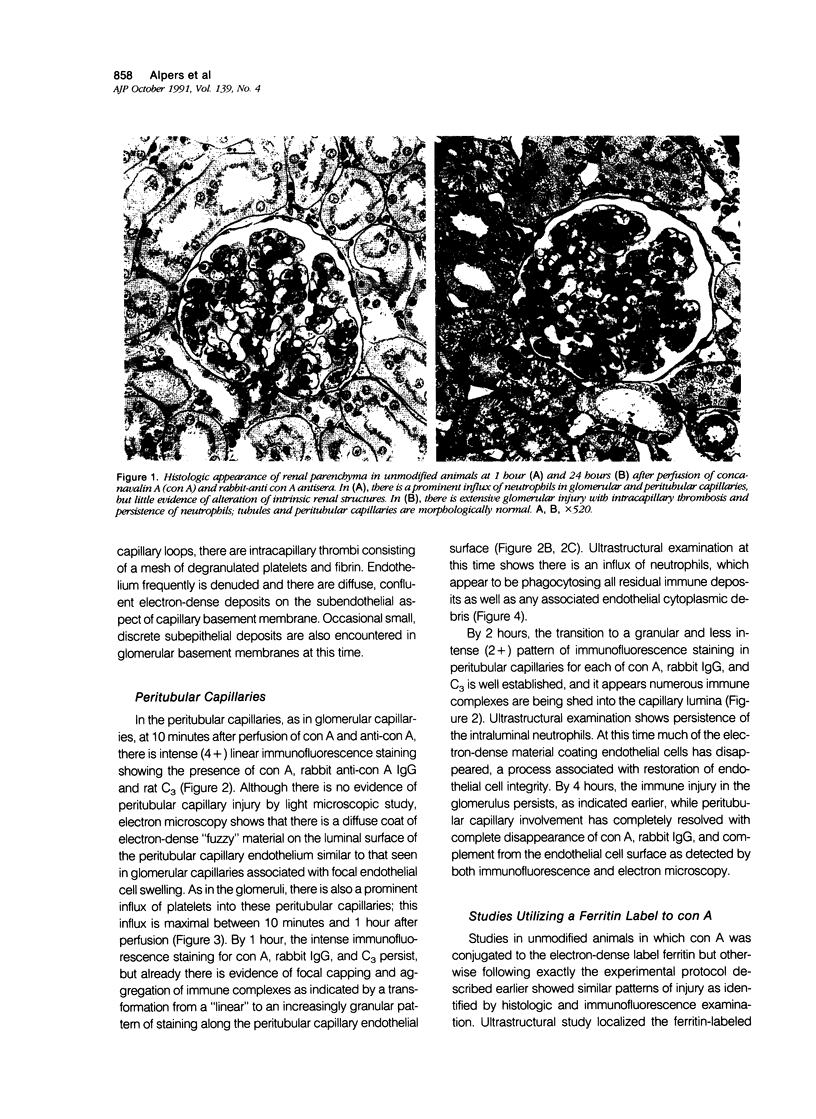
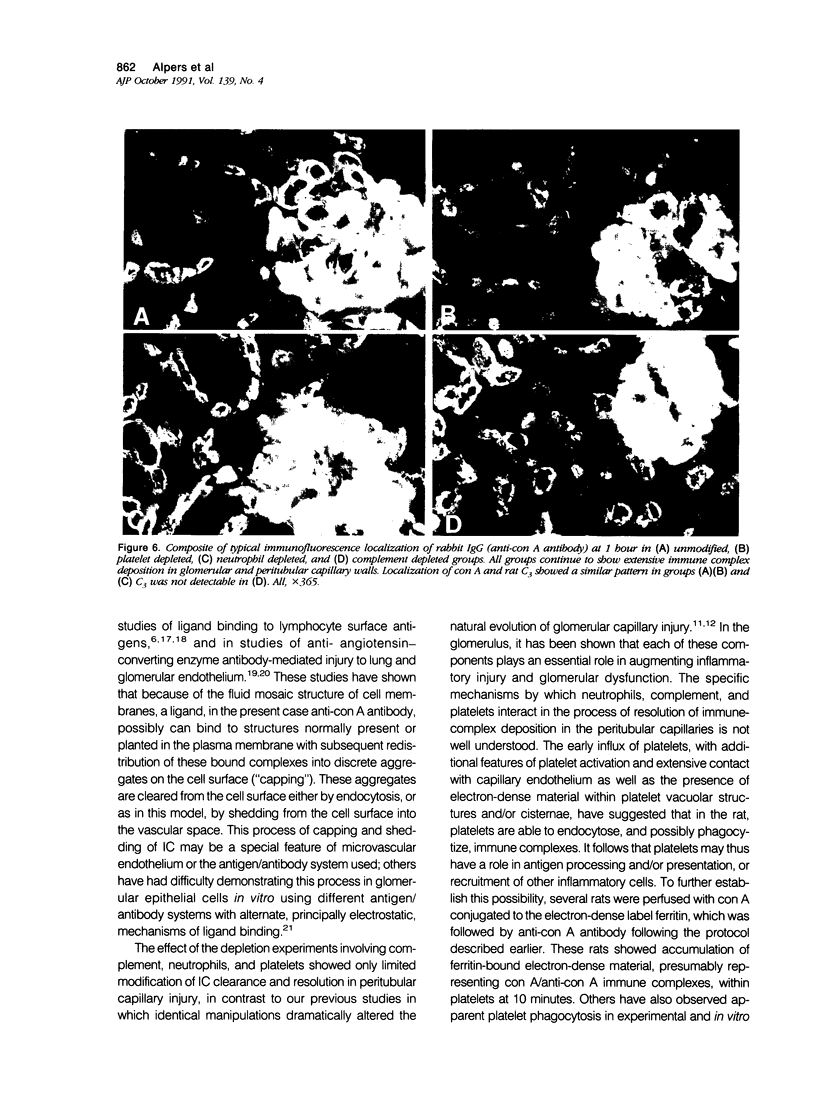
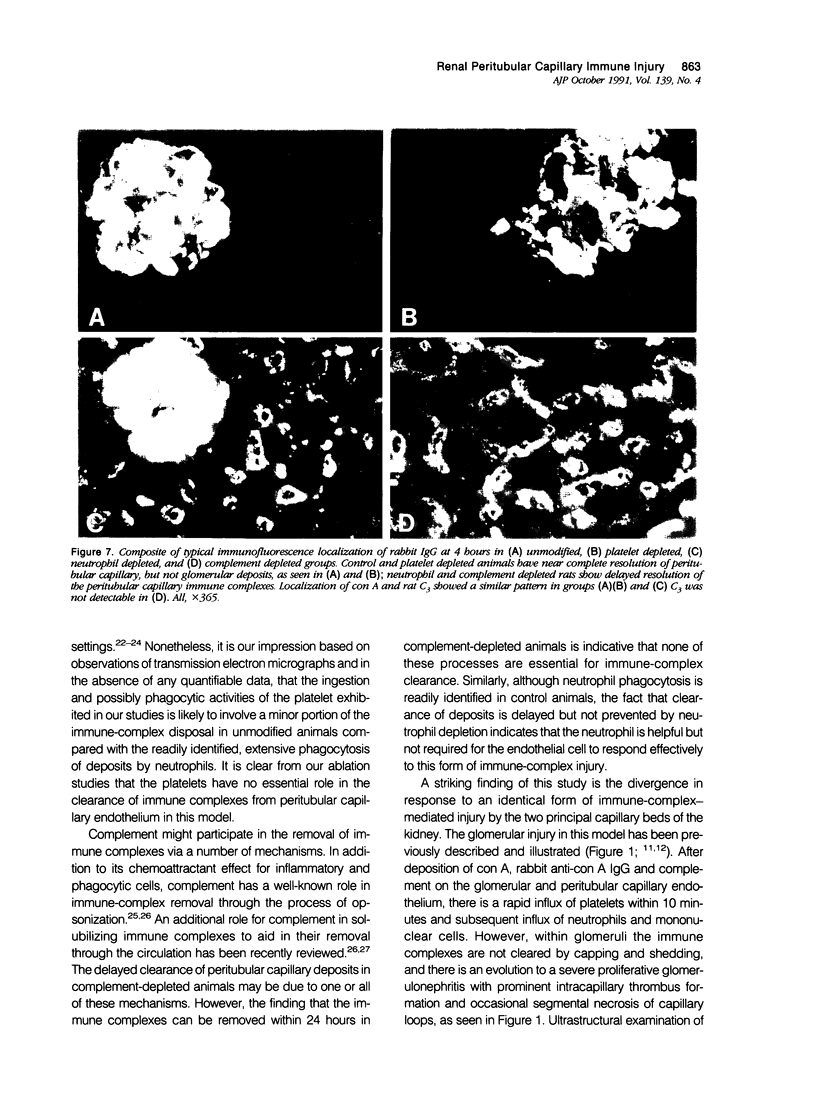
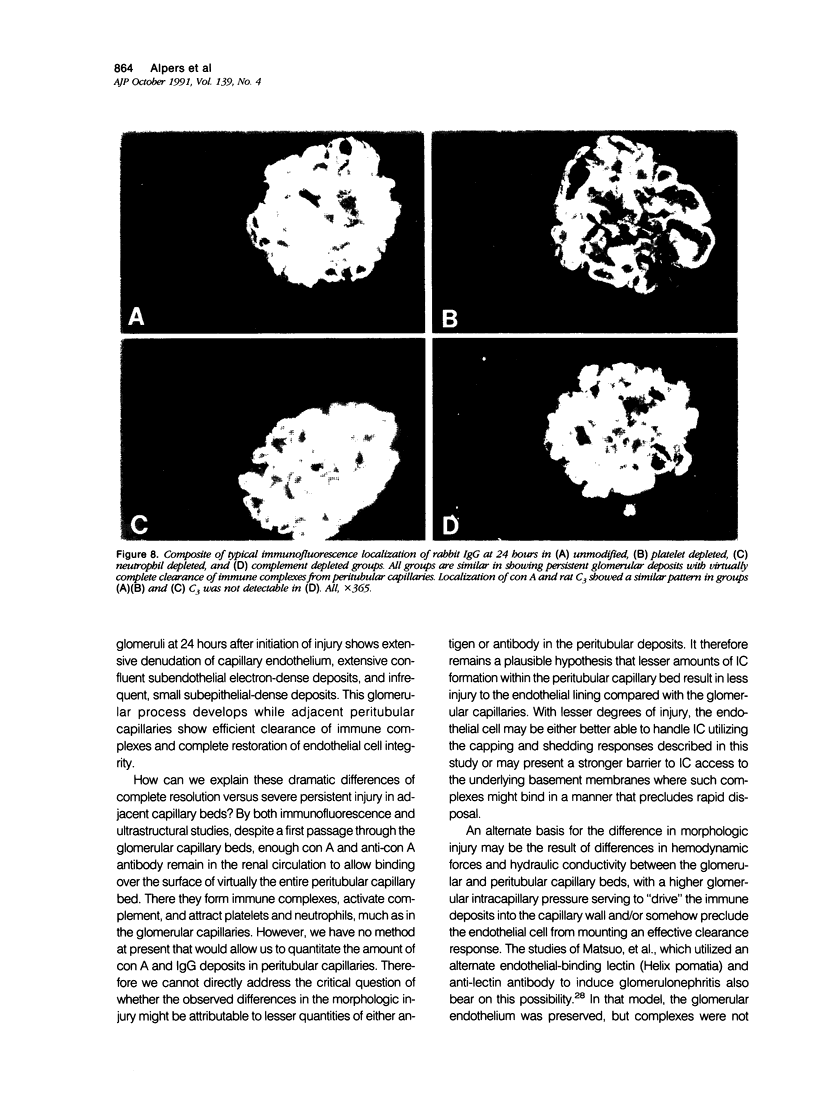
These experiments evaluated extraglomerular sites of renal immune complex (IC) deposition and specific features of host capability to remove these IC. Ex vivo perfusion of rat kidneys with the endothelium binding lectin concanavalin A (con A) followed by rabbit anti con A IgG results in a subendothelial IC nephritis in glomerular capillaries (GC) and diffuse IC formation with complement (C3) deposition in peritubular capillaries (PC). Histologic, immunofluorescence, and ultrastructural studies were performed at 10 minutes and 1, 4, and 24 hours after perfusion. At 10 minutes, strong linear binding of con A, rabbit IgG, and rat C3 to the endothelium was detected by immunofluorescence in both GC and PC. In GC this was followed by endothelial cell swelling and denudation (1 hour) with platelet and neutrophil infiltration and formation of subendothelial IC deposits which persisted at 4 and 24 hours. In contrast, some PC endothelial swelling was also present at 10 minutes and 1 hour, but ICs (IgG, con A, C3) were capped and shed into capillary lumina at 1 to 2 hours with complete clearance of IC by 4 hours. Selective neutrophil depletion, by antisera and irradiation, and complement depletion with cobra venom factor, delayed clearance of PC IC by several hours but complete clearance of IC with restored structural integrity of PC was still achieved by 24 hours. Platelet depletion had no effect on PC IC clearance. These studies demonstrate a model for study of PC IC. Such a model may aid our understanding of lupus nephritis in which extensive GC IC deposits associated with severe inflammatory injury may coexist with PC deposits. Efficient clearance of IC in PC compared with GC may be due to differences in hemodynamic forces, amounts of IC formed in each of these sites, differences in binding of IC to subendothelial basement membrane, or phenotypic specialization of the endothelium lining these two different capillary beds.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpers C. E., Beckstead J. H. Enzyme histochemistry in plastic-embedded sections of normal and diseased kidneys. Am J Clin Pathol. 1985 May;83(5):605–612. doi: 10.1093/ajcp/83.5.605. [DOI] [PubMed] [Google Scholar]
- Barba L. M., Caldwell P. R., Downie G. H., Camussi G., Brentjens J. R., Andres G. Lung injury mediated by antibodies to endothelium. I. In the rabbit a repeated interaction of heterologous anti-angiotensin-converting enzyme antibodies with alveolar endothelium results in resistance to immune injury through antigenic modulation. J Exp Med. 1983 Dec 1;158(6):2141–2158. doi: 10.1084/jem.158.6.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop G. A., Waugh J. A., Landers D. V., Krensky A. M., Hall B. M. Microvascular destruction in renal transplant rejection. Transplantation. 1989 Sep;48(3):408–414. doi: 10.1097/00007890-198909000-00011. [DOI] [PubMed] [Google Scholar]
- Bohle A., von Gise H., Mackensen-Haen S., Stark-Jakob B. The obliteration of the postglomerular capillaries and its influence upon the function of both glomeruli and tubuli. Functional interpretation of morphologic findings. Klin Wochenschr. 1981 Sep 15;59(18):1043–1051. doi: 10.1007/BF01747747. [DOI] [PubMed] [Google Scholar]
- Braun J., Unanue E. R. Surface immunoglobulin and the lymphocyte cytoskeleton. Fed Proc. 1983 May 15;42(8):2446–2451. [PubMed] [Google Scholar]
- Brentjens J. R., Andres G. Interaction of antibodies with renal cell surface antigens. Kidney Int. 1989 Apr;35(4):954–968. doi: 10.1038/ki.1989.79. [DOI] [PubMed] [Google Scholar]
- Brentjens J. R., Sepulveda M., Baliah T., Bentzel C., Erlanger B. F., Elwood C., Montes M., Hsu K. C., Andres G. A. Interstitial immune complex nephritis in patients with systemic lupus erythematosus. Kidney Int. 1975 May;7(5):342–350. doi: 10.1038/ki.1975.47. [DOI] [PubMed] [Google Scholar]
- Burke J. S., Simon G. T. Electron microscopy of the spleen. II. Phagocytosis of colloidal carbon. Am J Pathol. 1970 Jan;58(1):157–181. [PMC free article] [PubMed] [Google Scholar]
- Camussi G., Salvidio G., Niesen N., Brentjens J., Andres G. Effect of chlorpromazine on the development of experimental glomerulonephritis and Arthus reaction. Am J Pathol. 1988 Jun;131(3):418–434. [PMC free article] [PubMed] [Google Scholar]
- Cotran R. S. American Association of Pathologists president's address. New roles for the endothelium in inflammation and immunity. Am J Pathol. 1987 Dec;129(3):407–413. [PMC free article] [PubMed] [Google Scholar]
- Fries J. W., Mendrick D. L., Rennke H. G. Determinants of immune complex-mediated glomerulonephritis. Kidney Int. 1988 Sep;34(3):333–345. doi: 10.1038/ki.1988.186. [DOI] [PubMed] [Google Scholar]
- Golbus S. M., Wilson C. B. Experimental glomerulonephritis induced by in situ formation of immune complexes in glomerular capillary wall. Kidney Int. 1979 Aug;16(2):148–157. doi: 10.1038/ki.1979.116. [DOI] [PubMed] [Google Scholar]
- Johnson R. J., Alpers C. E., Pritzl P., Schulze M., Baker P., Pruchno C., Couser W. G. Platelets mediate neutrophil-dependent immune complex nephritis in the rat. J Clin Invest. 1988 Oct;82(4):1225–1235. doi: 10.1172/JCI113720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. J., Alpers C. E., Pruchno C., Schulze M., Baker P. J., Pritzl P., Couser W. G. Mechanisms and kinetics for platelet and neutrophil localization in immune complex nephritis. Kidney Int. 1989 Nov;36(5):780–789. doi: 10.1038/ki.1989.263. [DOI] [PubMed] [Google Scholar]
- Johnson R. J., Klebanoff S. J., Ochi R. F., Adler S., Baker P., Sparks L., Couser W. G. Participation of the myeloperoxidase-H2O2-halide system in immune complex nephritis. Kidney Int. 1987 Sep;32(3):342–349. doi: 10.1038/ki.1987.215. [DOI] [PubMed] [Google Scholar]
- Lehman D. H., Wilson C. B., Dixon F. J. Extraglomerular immunoglobulin deposits in human nephritis. Am J Med. 1975 Jun;58(6):765–796. doi: 10.1016/0002-9343(75)90632-4. [DOI] [PubMed] [Google Scholar]
- Magil A. B., Tyler M. Tubulo-interstitial disease in lupus nephritis. A morphometric study. Histopathology. 1984 Jan;8(1):81–87. doi: 10.1111/j.1365-2559.1984.tb02324.x. [DOI] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Matsuo S., Fukatsu A., Taub M. L., Caldwell P. R., Brentjens J. R., Andres G. Glomerulonephritis induced in the rabbit by antiendothelial antibodies. J Clin Invest. 1987 Jun;79(6):1798–1811. doi: 10.1172/JCI113021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo S., Yoshida F., Yuzawa Y., Hara S., Fukatsu A., Watanabe Y., Sakamoto N. Experimental glomerulonephritis induced in rats by a lectin and its antibodies. Kidney Int. 1989 Dec;36(6):1011–1021. doi: 10.1038/ki.1989.295. [DOI] [PubMed] [Google Scholar]
- Movat H. Z., Weiser W. J., Glynn M. F., Mustard J. F. Platelet phagocytosis and aggregation. J Cell Biol. 1965 Dec;27(3):531–543. doi: 10.1083/jcb.27.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M. H., D'Agati V., Appel G. B., Pirani C. L. Tubulointerstitial disease in lupus nephritis: relationship to immune deposits, interstitial inflammation, glomerular changes, renal function, and prognosis. Nephron. 1986;44(4):309–319. doi: 10.1159/000184012. [DOI] [PubMed] [Google Scholar]
- Paul L. C., van Es L. A., van Rood J. J., van Leeuwen A., de la Rivière G. B., de Graeff J. Antibodies directed against antigens on the endothelium of peritubular capillaries in patients with rejecting renal allografts. Transplantation. 1979 Mar;27(3):175–179. doi: 10.1097/00007890-197903000-00007. [DOI] [PubMed] [Google Scholar]
- Pober J. S., Cotran R. S. The role of endothelial cells in inflammation. Transplantation. 1990 Oct;50(4):537–544. doi: 10.1097/00007890-199010000-00001. [DOI] [PubMed] [Google Scholar]
- Renkonen R., Turunen J. P., Rapola J., Häyry P. Characterization of high endothelial-like properties of peritubular capillary endothelium during acute renal allograft rejection. Am J Pathol. 1990 Sep;137(3):643–651. [PMC free article] [PubMed] [Google Scholar]
- Salant D. J., Belok S., Madaio M. P., Couser W. G. A new role for complement in experimental membranous nephropathy in rats. J Clin Invest. 1980 Dec;66(6):1339–1350. doi: 10.1172/JCI109987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schifferli J. A., Ng Y. C., Peters D. K. The role of complement and its receptor in the elimination of immune complexes. N Engl J Med. 1986 Aug 21;315(8):488–495. doi: 10.1056/NEJM198608213150805. [DOI] [PubMed] [Google Scholar]
- Schreiner G. F., Unanue E. R. Membrane and cytoplasmic changes in B lymphocytes induced by ligand-surface immunoglobulin interaction. Adv Immunol. 1976;24:37–165. doi: 10.1016/s0065-2776(08)60329-6. [DOI] [PubMed] [Google Scholar]
- Schwartz M. M., Fennell J. S., Lewis E. J. Pathologic changes in the renal tubule in systemic lupus erythematosus. Hum Pathol. 1982 Jun;13(6):534–547. doi: 10.1016/s0046-8177(82)80268-2. [DOI] [PubMed] [Google Scholar]
- Tucker B. J., Gushwa L. C., Wilson C. B., Blantz R. C. Effect of leukocyte depletion on glomerular dynamics during acute glomerular immune injury. Kidney Int. 1985 Jul;28(1):28–35. doi: 10.1038/ki.1985.114. [DOI] [PubMed] [Google Scholar]
- Turner R. R., Beckstead J. H., Warnke R. A., Wood G. S. Endothelial cell phenotypic diversity. In situ demonstration of immunologic and enzymatic heterogeneity that correlates with specific morphologic subtypes. Am J Clin Pathol. 1987 May;87(5):569–575. doi: 10.1093/ajcp/87.5.569. [DOI] [PubMed] [Google Scholar]