Abstract
Abscisic acid (ABA) is a plant hormone involved in seed development and germination and in responses to various environmental stresses. The last step of ABA biosynthesis involves oxidation of abscisic aldehyde, and aldehyde oxidase (EC 1.2.3.1) is thought to catalyze this reaction. An aldehyde oxidase isoform, AOδ, encoded by AAO3, one of four Arabidopsis aldehyde oxidase genes (AAO1, AAO2, AAO3, and AAO4), is the most likely candidate for the enzyme, because it can efficiently catalyze the oxidation of abscisic aldehyde to ABA. Here, we report the isolation and characterization of an ABA-deficient Arabidopsis mutant that maps at the AAO3 locus. The mutant exhibits a wilty phenotype in rosette leaves, but seed dormancy is not affected. ABA levels were significantly reduced in the mutant leaves, explaining the wilty phenotype in rosettes, whereas the level in the mutant seeds was less reduced. No AOδ activity could be detected in the rosette leaves of the mutant. Sequence data showed that the mutant contains a G to A substitution in the AAO3 gene. The mutation causes incorrect splicing of the ninth intron of AAO3 mRNA. We thus conclude that the ABA-deficient mutant is impaired in the AAO3 gene and that the gene product, AOδ, is an aldehyde oxidase that catalyzes the last step of ABA biosynthesis in Arabidopsis, specifically in rosette leaves. Other aldehyde oxidases may be involved in ABA biosynthesis in other organs.
Abscisic acid (ABA) is a plant hormone that plays an important role in many aspects of plant growth and development, including seed maturation and dormancy as well as adaptation to a variety of environmental stresses (1). The regulation of these physiological processes is caused by de novo synthesis of ABA. Thus, the establishment of the ABA biosynthetic pathway and the isolation of the related gene(s) are essential for determining the role of ABA.
Recently, genes encoding ABA biosynthetic enzymes have been cloned, which has led to a better understanding of the regulation of ABA biosynthesis (2–4). The most important advances were the isolation of a gene for zeaxanthin epoxidase (ZEP), using the Nicotiana plumbaginifolia mutant aba2 (5) and the isolation of the 9-cis-epoxycarotenoid dioxygenase (NCED) gene, using the maize mutant vp14 (6, 7). ZEP converts zeaxanthin to violaxanthin by a two-step epoxidation, and NCED catalyzes the oxidative cleavage of 9-cis-xanthophylls to form xanthoxin. NCED cDNAs also were cloned from Phaseolus (8), tomato (9), and cowpea (10). It was shown that NCED, but not ZEP, expression is up-regulated in water-stressed leaves, indicating a regulatory role for NCED in ABA biosynthesis. It was proposed that there is a NCED gene family in plants and therefore the different NCED genes might be responsible for regulation of ABA biosynthesis in different tissues and under different environmental conditions (8).
The recent findings established that zeaxanthin epoxidase and NCED are located in plastids and the product of the NCED reaction, xanthoxin, is converted to ABA in the cytosol by two oxidation steps, via abscisic aldehyde (4). Several mutants related to the final step of ABA biosynthesis, the oxidation of abscisic aldehyde to ABA, have been isolated. These include the Arabidopsis aba3 mutant, the aba1 mutant of N. plumbaginifolia, and tomato flacca and sitiens mutants. However, these mutants, except for sitiens, are not impaired in abscisic aldehyde oxidase, but are deficient in the synthesis of the molybdenum cofactor (Moco), which is necessary for aldehyde oxidase activity (11–15). Only the sitiens mutant of tomato is thought to have a mutation in a structural gene of an aldehyde oxidase specific for abscisic aldehyde (11, 16). Although three putative aldehyde oxidase cDNAs have been cloned from tomato (17, 18), the corresponding gene for sitiens has not yet been identified.
The existence of aldehyde oxidase isoforms has been reported in several plants (17, 19–22). Our previous work demonstrated the presence of three aldehyde oxidase isoforms, AOα, AOβ, and AOγ, in extracts of Arabidopsis seedlings by activity staining after native PAGE (21). They had relatively wide substrate specificity, but abscisic aldehyde was a poor substrate for all three. Subsequently, we cloned four Arabidopsis aldehyde oxidase cDNAs (AAO1, AAO2, AAO3, and AAO4, formerly called atAO-1, atAO-2, atAO-3, and atAO-4, respectively) (23). AtAO1, AtAO2, and AtAO3, corresponding to AAO1, AAO4, and AAO2, respectively, also were cloned independently by Hoff et al. (24). We also found an aldehyde oxidase isoform, AOδ, which is encoded by AAO3. This isoform efficiently oxidizes abscisic aldehyde to ABA in rosette leaves. AAO3 mRNA was abundant in the rosette leaves, and the expression was up-regulated by dehydration (25). However, our preliminary experiments showed no significant increase in enzyme activity and AAO3 protein level after desiccation, indicating that the oxidation of abscisic aldehyde is not a limiting step in ABA biosynthesis in leaves as already shown by Sindhu and Walton (26). In this study, we describe an ABA-deficient mutant of Arabidopsis defective in the AAO3 gene. Characterization of the mutant demonstrated that AOδ is an abscisic aldehyde oxidase that is predominantly involved in ABA biosynthesis in leaves.
Materials and Methods
Mutant Isolation and Genetic Characterization.
Seeds of the abi3–1 mutant [in Landsberg erecta (Ler) genetic background] were imbibed in a solution of 15 mM ethyl methanesulfonate. The M2 population that was described before in Ooms et al. (27) was screened for wilty plants. Plant growth and germination experiments were performed as described by Léon-Kloosterziel et al. (28). Water loss was determined by weighing well-watered plants that had just started to bolt. The rosettes were cut from their roots, placed on filter paper on a bench at ambient temperature, and weighed every half-hour.
Mapping was performed on F3 lines derived from the cross of the aao3 mutant with wild-type Columbia that were phenotypically classified as having a slightly wilty or wild-type phenotype. F3 lines segregating for the mutation were not included in the analysis. Cosegregation of the mutant and the AAO3 gene was tested with the cleaved amplified polymorphic sequence (CAPs) marker described by Sekimoto et al. (23).
ABA Determinations.
Plant material was grown as before (29) and used for ABA determinations when still in the rosette stage. ABA was extracted and purified as described (30). Quantification of methyl-ABA was performed with a Hewlett–Packard 6890 gas chromatograph, equipped with an electron capture detector, and using endrin as an internal standard.
Enzyme Extraction and Activity Stain.
For activity determination, Northern blotting, and Western blotting, Arabidopsis thaliana Ler wild-type and mutant seeds were sown in pots containing vermiculite and watered with nutrient solution under 16 h light and 8 h darkness at 22°C for about 2 months. Enzyme extraction and activity staining after native PAGE were carried out essentially according to the methods described (20). Plant tissue was homogenized in 8 ml/g fresh weight (FW) extraction buffer [50 mM Tris⋅HCl, pH 7.5/1 mM EDTA/1 μM sodium molybdate/10 μM FAD/2 mM DTT/one tablet/100 ml protease inhibitor (Complete Protease inhibitor mixture tablets, Roche Diagnostics) and Polyclar AT (0.2 g/g FW)]. The extract was fractionated with ammonium sulfate (0–60% saturation), and excess protein was removed by heat treatment (3 min at 60°C). After electrophoresis with 7.5% native acrylamide gel at 4°C, activity bands of aldehyde oxidase were developed with aldehyde substrate (200 μM) at 30°C in the dark for 30–60 min.
Sequencing of the AAO3 Gene.
Genomic DNA was isolated from Ler and the mutant using Plant DNA ZOL Reagent (GIBCO/BRL) according to the manufacturer's instructions. Based on the sequence of AAO3 gene from Columbia background (AC007154) reported by the genome project (http://www.ncbi.nlm.nih.gov/htbin-post/Entrez/query?uid=AC007154&form=6&db=n&Dopt=gDopt=g), primers 5′-TGTGTATGTTGATACAAGAGAGT-3′ and 5′-GGTTTTGAAACCATTAGTTATGC-3′, corresponding to nucleotides 2453–2475 and 10258–10280 of AC007154, respectively, were designed to amplify 7.8 kb of the AAO3 genomic region, including 2.6 kb of the 5′ upstream sequences. The PCR fragments were cloned into the pCR2.1 vector (Invitrogen), and several independent clones were sequenced and compared between Ler and the mutant in three independent experiments. The wild-type AAO3 clone was used for complementation of the mutant.
RNA Extraction, Reverse Transcription–PCR (RT-PCR), and Northern Blotting.
Total RNA was extracted by the method described by Verwoerd et al. (31), except that the buffer composition was 100 mM Tris⋅HCl, pH 8.0/100 mM LiCl/10 mM EDTA/1% SDS. For RT-PCR, first-strand cDNA was synthesized by using the superscript preamplification system (GIBCO/BRL). PCR was performed by using oligonucleotide primers 5′-TACACTAGGTATGATCCAAGGAG-3′ and 5′-ACACTATACAATCCGCAAAGAGA-3′, designed at the positions corresponding to nucleotides 3423–3445 and 4138–4160 of AAO3 cDNA (AB016622), respectively, to amplify a fragment of 736-bp wild-type AAO3 cDNA. Northern blotting was carried out by using 10 μg total RNA. RNA samples were loaded on a 1.5% agarose gel containing formaldehyde and Mops [3-(N-morpholino)propanesulfonic acid] buffer and transferred to a nylon membrane. Hybridization was performed by using the 32P-dCTP-labeled AAO3 full-length cDNA and a 367-bp cDNA fragment of APETALA2 (AP2) digested with BamHI and XhoI as probes under highly stringent conditions (32). No cross-hybridization occurred, because the probe showed a specific pattern of the hybridization between four AAO probes (25).
Western Blotting.
Anti-AAO3 antibodies were obtained as described (25). Western blotting was performed by using a Vectastain Elite ABC kit (Vector Laboratories) diluted 100-fold in TBS (20 mM Tris⋅HCl, pH 7.5/150 mM NaCl) containing 0.05% Tween-20 according to the previous study (20). Peroxidase activity was visualized by staining with an immunostain HRP-1000 kit (Konika, Tokyo).
Complementation.
AAO3 genomic DNA obtained as described above was excised and cloned into transformation vector pPZP211 (33). Transformation of the mutant was performed according to the procedure described by Bechtold et al. (34).
Results
Isolation of a Wilty Mutant, aao3, of Arabidopsis.
Screening of M2 plants derived from ethyl methanesulfonate mutagenesis of the abi3–1 mutant under greenhouse conditions led to the identification of a mutant with a mild wilty phenotype. This mutant, named aao3 for reasons explained hereafter, was backcrossed to the Ler wild type and in the segregating progeny, F3 lines with a wilty phenotype, but lacking the abi3–1 mutation, were identified. ABA determinations of rosettes (Table 1) indicated that this mutant has a reduced ABA content. Seed germination experiments with the homozygous wilty mutant indicated that, in contrast to all known wilty ABA-deficient and ABA-insensitive mutants in Arabidopsis (35), the seeds had dormancy comparable to that of Ler. This finding suggested that the mutant represented a novel locus affected in ABA biosynthesis or catabolism. This was further confirmed by the complementation of the wilty phenotype in F1 hybrids derived from crossing the mutant to the ABA-deficient mutants, aba1, aba2, and aba3 (data not shown).
Table 1.
ABA levels (μg/g dry weight) in rosettes, siliques, and seeds of wild type and two ABA-deficient mutants
| Genotype | Turgid rosettes | Wilted rosettes | Immature siliques | Mature dry seeds |
|---|---|---|---|---|
| Wild type (Ler) | 0.35 | 2.20 | 0.59 | 0.24 |
| aao3 | 0.13 | 0.46 | 0.25 | 0.14 |
| aba3-2 | nd | nd | 0.10 | 0.03 |
nd = not determined. In earlier measurements (28) stressed leaves of aba3-2 contained only 8% of the ABA content in Ler stressed rosettes. All measurements were repeated once with similar results.
ABA Levels in Wild-Type and Mutant Plants.
ABA levels were determined in turgid and water-stressed rosette leaves, immature siliques, and mature dry seeds (Table 1). In the turgid rosettes of the aao3 mutant, the ABA level was about one-third of that in wild type and the increase after water stress was much less than in wild-type rosettes. In siliques and dry seeds of aao3 plants, the amount of ABA was less reduced (about one-half of wild type) compared with the reduction in rosettes. Immature siliques and dry seeds of aba3–2 mutant, deficient in Moco that is required for aldehyde oxidase activity (13), contained a more reduced amount of ABA (1/6 to 1/10 of wild type).
Characterization of aao3 Mutant Phenotype.
The mutant was characterized physiologically in comparison to the previously described aba3–2 mutant (28) and the double mutants of aao3 with abi3–1 and aba3–2 by (i) determination of water loss in detached rosettes, and (ii) measurement of the loss of seed dormancy caused by after-ripening.
The results in Fig. 1 indicate that the enhanced water loss characteristics of aao3 are comparable to those of the aba3–2 mutant, which has a smaller vegetative mass than aao3. However, plants of the double mutant aba3–2 aao3 have a very weak growth habit, which correlates with a much more rapid water loss by detached rosettes. Results of germination experiments (Fig. 2A) confirmed that seeds of the aao3 mutant have dormancy similar to that of Ler seeds when tested in white light. However, when tested in darkness, aao3 seeds germinated slightly better than wild-type seeds (Fig. 2B). Some further reduction in dormancy over that of abi3–1 was observed in the double mutant, abi3–1 aao3. However, the double mutant did not have the green seed phenotype observed for the aba3–abi3 double mutant (28). This almost normal phenotype for dormancy in light coincided with a reduction in ABA content both during seed development and in mature seeds (Table 1). However, the reduction in ABA levels observed in these tissues was less than in leaves.
Figure 1.
Water loss of wild type (◊), aba3–2 (▵), aao3 (□), and aba3–2 aao3 (▴) expressed as loss of fresh weight. (Inset) The average fresh weight of the genotypes is shown. WT, wild type.
Figure 2.
Germination percentage of wild type (◊), aba3–2 (▵), aao3 (□), abi3–1 (○), aba3–2 aao3 (▴), and abi3–1 aao3 (●) seeds in the light (A) and darkness (B).
Mapping of the Mutant.
Mapping of the mutant revealed that the gene was located on chromosome 2, very close to the erecta mutation, where no previously known ABA-deficient mutants had been mapped. This position is similar to that of AAO3, which was shown to encode an abscisic aldehyde oxidase expressed in Arabidopsis leaves (25). The nearly full-length AAO3 cDNA (AB016622) exhibits a 198-nt 5′ untranslated region, followed by a 3,999-nt ORF and a 121-nt 3′ untranslated sequence. The ORF of the cDNA predicts a protein of 1,332 aa with molecular weight of 146,665, and the protein contains the conserved sequence for two iron-sulfur centers and five motifs involved in Moco binding. Colocation of the mutant and AAO3 polymorphism described before (23) was confirmed by testing 22 homozygous mutant and 13 homozygous wild-type F3 lines derived from the cross, aao3 × Columbia. In all 35 lines aao3 cosegregated with the morphological erecta marker. This finding is in agreement with the location of AAO3, 378 kbp south of erecta (data obtained from the TIGR database; http://www.tigr.org/tdb/ath1/htmls/).
Abscisic Aldehyde Oxidase in the Mutant and Wild-Type Plants.
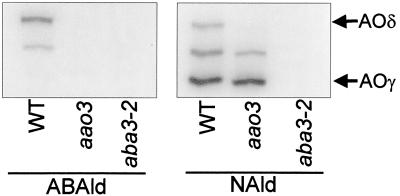
We next investigated activities of aldehyde oxidase, including the AOδ isoform encoded by AAO3, both in wild-type and aao3 plants. The activity also was checked in aba3–2 plants, a mutant lacking all aldehyde oxidase activities because of its Moco deficiency (Fig. 3). In rosette leaves of wild-type plants, an intense band of AOδ was detected with the abscisic aldehyde substrate. In contrast, the aao3 and aba3–2 mutants lacked the activity. When 1-naphthaldehyde was used as a substrate, the aao3 mutant exhibited two activity bands other than AOδ that also were detected in wild type, whereas the aba3–2 mutant lacked all activities. This indicated that aao3 is specifically impaired in the activity of AOδ. Almost no abscisic aldehyde oxidase activity was detected in siliques, dry seeds, and imbibed seeds of all three genotypes (data not shown). The band observed at the middle position in Fig. 3 probably represents AOβ, a heterodimer of AAO1 and AAO2 (36), and/or another isoform originating as a heterodimer consisting of AAO2 and AAO3 products as discussed by Seo et al. (25).
Figure 3.
Aldehyde oxidase activities in the rosette leaves of aao3, aba3–2, and wild-type (WT) Arabidopsis. Enzyme extracts were subjected to native PAGE, and activity bands were developed by using abscisic aldehyde (ABAld) or 1-naphthaldehyde (NAld). Each lane was loaded with 65 μg of protein.
Detection of a Mutation in AAO3 in the aao3 Mutant.
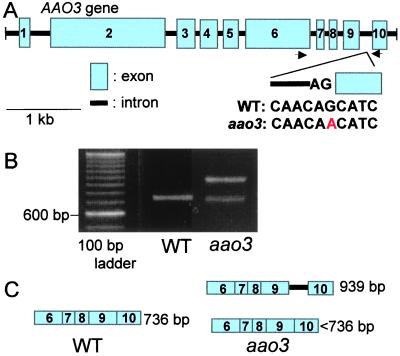
To determine whether the aao3 mutant contains a mutation in the AAO3 gene, genomic DNAs of AAO3 from Ler wild type and the mutant were sequenced. Six differences in the nucleotide sequences were found between the AAO3 genes from Columbia and Ler backgrounds. The differences between Columbia and Ler in their sequences are as follows; A and C, C and G, T and G, G and T, T and C, and T and G at nucleotide positions 5127, 5412, 5429, 5917, 6133, and 6803 of the Columbia AAO3 gene (AC007154). In the aao3 mutant, a single base pair substitution was found at the end of the ninth intron of the AAO3 gene (Fig. 4A). The mutation causes a transition of the conserved 3′ splice site AG to AA. It is expected that the mutation results in incorrect splicing of the intron. RT-PCR was performed by using total RNA prepared from rosettes of wild type and aao3 mutant (Fig. 4B). Primers were designed to amplify 736-bp AAO3 cDNA fragments of wild type including the region where the ninth intron had been spliced out. A cDNA fragment of the correct size was detected only in wild-type rosette leaves. cDNA fragments of two different sizes were found in aao3 leaves. One fragment had a size of around 950 bp, which corresponds to the size of the 736-bp fragment plus the 203 bp of the ninth intron. The other was slightly smaller than the 736-bp fragment (Fig. 4C). The RT-PCR fragments obtained from aao3 mutant RNA were cloned into the sequencing vector, and three independent clones of the longer fragment and 13 clones of the shorter fragments than wild-type DNA were sequenced. The longer fragments contained the entire ninth intron in their sequence. Three different sequences were detected among the shorter fragments. Incorrect splicing had resulted in shorter transcripts by 2, 6, or 30 bp compared with wild type and no correctly spliced sequence was detected. The incorrect splicing caused frameshifts, or deletion of amino acids. These frameshifts or deletions occurred in one of the Moco binding sites “SGEPPL” in the AAO3 gene (23). The results indicate that there are hardly any correct mRNA transcripts in aao3 plants and that, if the translation from this incorrectly spliced mRNA proceeds to some extent, the protein cannot effectively oxidize abscisic aldehyde to ABA.
Figure 4.
Structure and expression of AAO3 and aao3. (A) Structure of the AAO3 gene and location of the mutation at the end of the ninth intron. Arrows indicate the position where primers for RT-PCR were designed. WT, wild type. (B) RT-PCR fragments obtained from cDNAs synthesized from total RNA prepared from rosette leaves using primers represented in A. (C) Illustration of RT-PCR fragments detected in wild type and aao3.
Expression of AAO3 in Rosette Leaves.
Expression of AAO3 mRNA and AAO3 protein in the rosette leaves of wild type and aao3 mutant were analyzed by Northern and Western blotting, respectively. AAO3 transcripts were detected both in the wild type and aao3 mutants. However, a more intense signal was observed in the mutants, whereas AP2 mRNA expressed ubiquitously in plant organs (37) was detected at the same level in both wild-type and mutant leaves (Fig. 5A). Western blotting, using anti-AAO3 antibodies, revealed that AOδ is not present in detectable amounts in extract from leaves of mutant plants (Fig. 5B).
Figure 5.
Expression of AAO3 mRNA and protein in the rosettes of wild type (WT) and the aao3 mutant. (A) Total RNA was extracted from rosettes of 2-month-old plants. Ten micrograms of RNA was separated on 1.5% (wt/vol) agarose gels containing 5% (vol/vol) formaldehyde. Specific probes for AAO3 and AP2 were used for hybridization. (B) Immunoblotting with protein extracts from the rosette leaves of wild-type and aao3 plants. Crude enzyme extracts (60 μg protein) of rosettes were subjected to native PAGE followed by immunoblot analysis using anti-AAO3 antibodies. Arrow indicates the position of AOδ.
Complementation of the aao3 Mutant by the AAO3 Gene.
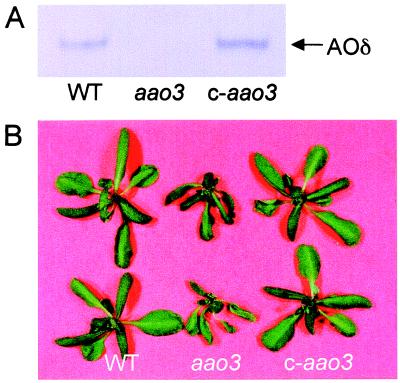
The AAO3 gene was introduced into aao3 mutant plants by Agrobacterium-mediated transformation. Transformants were selected for kanamycin resistance, and a T3 population that was homozygous for the transgene was obtained. Leaf extracts from these transgenic plants exhibited the activity of AOδ (Fig. 6A), and the wilty phenotype of aao3 mutant plants was restored to normal (Fig. 6B). We conclude from these results that the mutation in AAO3 causes the ABA deficiency of the mutant and that AOδ encoded by AAO3 is responsible for the last step in ABA biosynthesis in rosette leaves of Arabidopsis.
Figure 6.
Complementation of aao3 mutant. (A) Activity of AOδ in wild type (WT), aao3, and aao3 complemented with wild-type AAO3 gene (c-aao3) was developed by using abscisic aldehyde as a substrate. Each lane was loaded with 60 μg of protein. (B) Plants of wild type, aao3, and c-aao3 were cut from roots and left for 1 h at room conditions.
Discussion
Aldehyde oxidase is involved in ABA biosynthesis by catalyzing the last step of the pathway. Our recent results revealed that in Arabidopsis there is a gene family consisting of at least four aldehyde oxidase genes (23) and that one of the genes, AAO3, encodes AOδ, which has a high specificity for abscisic aldehyde (25). The AAO3 mRNA is expressed mainly in rosette leaves, and most of the AOδ enzyme activity is detected in leaf tissues. Thus, we proposed that AOδ is involved in ABA biosynthesis in Arabidopsis leaves and that other aldehyde oxidases are involved in ABA biosynthesis in other organs, such as roots, siliques, and seeds.
The present study shows that the ABA deficiency in aao3 is caused by a mutation in the AAO3 gene. The mutant exhibits a wilty phenotype in the leaves, but is not or hardly affected in seed dormancy. In fact, the ABA level is reduced in rosettes of aao3 compared with wild type, but a less-reduced ABA level is observed in siliques and seeds of aao3. Although a significantly reduced ABA level in siliques and seeds was observed, one explanation for the lack of a nondormant phenotype might be that the ABA levels in the aao3 mutant are above the threshold required for induction of dormancy. These observations lead to the following question: How is ABA synthesized in seeds of the aao3 mutant? One possibility is that the aao3 mutant is leaky. However, in leaky aba1 mutants of Arabidopsis, such as aba1–3, the reduction in ABA content is similar in seeds and leaves, and a clear nondormant phenotype is observed (38, 39). Furthermore, no detectable amounts of correctly transcribed mRNA, AOδ protein, and its activity were observed in aao3 rosette leaves (Figs. 3–5). Thus, it is unlikely that the ABA present in the aao3 mutant is synthesized by the remaining activity of AOδ. A second possibility is that other aldehyde oxidase(s) is able to oxidize abscisic aldehyde to produce ABA in this mutant. AAO4 is a good candidate for a gene encoding an enzyme, which is able to oxidize abscisic aldehyde, because its transcript is expressed mainly in siliques (25). However, the enzymatic nature of the AAO4 protein has not yet been determined.
As shown in Table 1, a significant residual level of ABA (37% and 20%) was detected in turgid and wilted rosettes of the aao3 mutant, respectively, even though no abscisic aldehyde oxidase activity was detected in the leaves. It is possible that ABA in the leaves of the aao3 mutant is imported from other organs, such as the roots. It also could be produced by other aldehyde oxidase isoforms and/or via the shunt pathway (40).
In tomato, abscisic aldehyde oxidase is most likely encoded by the Sitiens (Sit+) gene (11, 16). Plants of the sitiens (sit) mutant show more extreme symptoms of ABA deficiency and reduced growth than the flacca (flc) and notabilis (not) mutants because of excessive water loss (41). Thus, the severe phenotype of the sit mutant differs from the mild symptoms observed in the aao3 mutant. The two mutants also differ in that seed dormancy is not or hardly affected in aao3, whereas sit mutants are clearly nondormant (42). Because the sit mutant in tomato is more extreme it is possible that the Sit+-encoded aldehyde oxidase alone is responsible for the last step in ABA biosynthesis in all tissues.
Aldehyde oxidases require a Moco to which sulfur is added. Previously it was shown that the addition of sulfur is mediated by the ABA3 gene product in Arabidopsis (13). An additive effect for water loss in the aba3–2 and aao3 double mutant (Fig. 1) can be explained by the finding that the aao3 mutant is impaired only in the AOδ isoform, whereas the aba3–2 mutant, which itself has a “leaky” ABA deficiency (28), is impaired in all aldehyde oxidase isoforms as well. Water loss from excised rosettes of aba3–2 and aao3 was very similar, although plant vigor was much more reduced in the aba3–2 than in the aao3 mutant. This might be because ABA deficiency in aao3 is less extreme at this later stage of rosette development than at early stages, or it may be because of the pleiotropic effects of the Moco deficiency in the aba3–2 mutant.
The present data indicate that AOδ, the AAO3 product, catalyzes the last step of ABA biosynthesis only in rosette leaves. Transgenic lines, such as knockout null mutants and overexpression lines for each AAO gene, will be useful to elucidate the roles of the individual AAO genes in ABA biosynthesis in relation to plant development and environmental responses.
Acknowledgments
We thank Ms. Hetty Blankestijn-de Vries and Ms. Corrie Hanhart for their assistance with the experiments in Wageningen and Dr. Karen Léon-Kloosterziel for selecting the mutant. This work was supported in part by a Grant-in Aid for Scientific Research (B) 10559017 (to T.K.) from the Ministry of Education, Science, Sports and Culture, Japan, and by Fund for Research Fellowship of the Japan Society for the Promotion of Science for Young Scientist (to M.S.). A.M.-P., A.J.M.P, and M.K. were supported by Grant Bio4-TC96–0062 from the European Union. J.A.D.Z. was supported by U.S. Department of Energy Grant DE-FG02–91ER20021.
Abbreviations
- ABA
abscisic acid
- Moco
molybdenum cofactor
- NCED
9-cis-epoxycarotenoid dioxygenase
- RT-PCR
reverse transcription–PCR
- Ler
Landsberg erecta
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.220426197.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.220426197
References
- 1.Zeevaart J A D, Creelman R A. Annu Rev Plant Physiol Plant Mol Biol. 1988;39:439–473. [Google Scholar]
- 2.Liotenberg S, North H, Marion-Poll A. Plant Physiol Biochem. 1999;37:341–350. [Google Scholar]
- 3.Zeevaart J A D. In: Biochemistry and Molecular Biology of Plant Hormones. Hooykaas P J J, Hall M A, Libbenga K R, editors. Amsterdam: Elsevier; 1999. pp. 189–207. [Google Scholar]
- 4.Cutler A J, Krochko J E. Trends Plant Sci. 1999;4:472–478. doi: 10.1016/s1360-1385(99)01497-1. [DOI] [PubMed] [Google Scholar]
- 5.Marin E, Nussaume L, Quesada A, Gonneau M, Sotta B, Hugueney P, Frey A, Marion-Poll A. EMBO J. 1996;15:2331–2342. [PMC free article] [PubMed] [Google Scholar]
- 6.Schwartz S H, Tan B C, Gage D A, Zeevaart J A D, McCarty D R. Science. 1997;276:1872–1874. doi: 10.1126/science.276.5320.1872. [DOI] [PubMed] [Google Scholar]
- 7.Tan B C, Schwartz S H, Zeevaart J A D, McCarty D R. Proc Natl Acad Sci USA. 1997;94:12235–12240. doi: 10.1073/pnas.94.22.12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qin X, Zeevaart J A D. Proc Natl Acad Sci USA. 1999;96:15354–15361. doi: 10.1073/pnas.96.26.15354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson A J, Jackson A C, Parker R A, Morpeth D R, Burbidge A, Taylor I B. Plant Mol Biol. 2000;42:833–845. doi: 10.1023/a:1006448428401. [DOI] [PubMed] [Google Scholar]
- 10.Iuchi S, Kobayashi M, Yamaguchi-Shinozaki K, Shinozaki K. Plant Physiol. 2000;123:553–562. doi: 10.1104/pp.123.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taylor I B, Linforth R S T, Al-Naieb R J, Bowman W R, Marples B A. Plant Cell Environ. 1988;11:739–745. [Google Scholar]
- 12.Leydecker M-T, Moureaux T, Kraepiel Y, Schnorr K, Caboche M. Plant Physiol. 1995;107:1427–1431. doi: 10.1104/pp.107.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwartz S H, Léon-Kloosterziel K M, Koornneef M, Zeevaart J A D. Plant Physiol. 1997;114:161–166. doi: 10.1104/pp.114.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akaba S, Leydecker M-T, Moureaux T, Oritani T, Koshiba T. Plant Cell Physiol. 1998;39:1281–1286. [Google Scholar]
- 15.Mendel R R, Schwarz G. Crit Rev Plant Sci. 1999;18:33–69. [Google Scholar]
- 16.Marin E, Marion-Poll A. Plant Physiol Biochem. 1997;35:369–372. [Google Scholar]
- 17.Ori N, Eshed Y, Pinto P, Paran I, Zamir D, Fluhr R. J Biol Chem. 1997;272:1019–1025. doi: 10.1074/jbc.272.2.1019. [DOI] [PubMed] [Google Scholar]
- 18.Min, X., Okada, K., Brockmann, B., Koshiba, T, & Kamiya, Y. (2000) Biochim. Biophys. Acta, in press. [DOI] [PubMed]
- 19.Rothe G M. Plant Cell Physiol. 1974;15:493–499. [Google Scholar]
- 20.Koshiba T, Saito E, Ono N, Yamamoto N, Sato M. Plant Physiol. 1996;110:781–789. doi: 10.1104/pp.110.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seo M, Akaba S, Oritani T, Delarue M, Bellini C, Caboche M, Koshiba T. Plant Physiol. 1998;116:687–693. doi: 10.1104/pp.116.2.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Omarov R T, Akaba S, Koshiba T, Lips S H. J Exp Bot. 1999;50:63–69. [Google Scholar]
- 23.Sekimoto H, Seo M, Kawakami N, Komano T, Desloire S, Liotenberg S, Marion-Poll A, Caboche M, Kamiya Y, Koshiba T. Plant Cell Physiol. 1998;39:433–442. doi: 10.1093/oxfordjournals.pcp.a029387. [DOI] [PubMed] [Google Scholar]
- 24.Hoff T, Frandsen G I, Rocher A, Mundy J. Biochim Biophys Acta. 1998;1398:397–402. doi: 10.1016/s0167-4781(98)00085-2. [DOI] [PubMed] [Google Scholar]
- 25.Seo M, Koiwai H, Akaba S, Komano T, Oritani T, Kamiya Y, Koshiba T. Plant J. 2000;23:481–488. doi: 10.1046/j.1365-313x.2000.00812.x. [DOI] [PubMed] [Google Scholar]
- 26.Sindhu R K, Walton D C. Plant Physiol. 1987;85:916–921. doi: 10.1104/pp.85.4.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ooms J J J, Léon-Kloosterziel K M, Bartels D, Koornneef M, Karssen C M. Plant Physiol. 1993;102:1185–1191. doi: 10.1104/pp.102.4.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Léon-Kloosterziel K M, Alvarez-Gil M, Ruijs G J, Jacobsen S E, Olszewski N E, Schwartz S H, Zeevaart J A D, Koornneef M. Plant J. 1996;10:655–661. doi: 10.1046/j.1365-313x.1996.10040655.x. [DOI] [PubMed] [Google Scholar]
- 29.Rock C D, Zeevaart J A D. Proc Natl Acad Sci USA. 1991;88:7496–7499. doi: 10.1073/pnas.88.17.7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Léon-Kloosterziel K M, van de Bunt G A, Zeevaart J A D, Koornneef M. Plant Physiol. 1996;110:233–240. doi: 10.1104/pp.110.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verwoerd T C, Dekker B M M, Hoekema A. Nucleic Acids Res. 1989;17:2362. doi: 10.1093/nar/17.6.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 33.Hajdukiewicz P, Svab Z, Maliga P. Plant Mol Biol. 1994;25:989–994. doi: 10.1007/BF00014672. [DOI] [PubMed] [Google Scholar]
- 34.Bechtold N, Ellis J, Pelletier G. C R Acad Sci. 1993;316:1194–1199. [Google Scholar]
- 35.Koornneef M, Léon-Kloosterziel K M, Schwartz S H, Zeevaart J A D. Plant Physiol Biochem. 1998;36:83–89. doi: 10.1104/pp.114.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Akaba S, Seo M, Dohmae N, Takio K, Sekimoto H, Kamiya Y, Furuya N, Komano T, Koshiba T. J Biochem. 1999;126:395–401. doi: 10.1093/oxfordjournals.jbchem.a022463. [DOI] [PubMed] [Google Scholar]
- 37.Jofuku K D, den Boer B G W, Van Montagu M, Okamuro J K. Plant Cell. 1994;6:1211–1225. doi: 10.1105/tpc.6.9.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koornneef M, Jorna M L, Brinkhorst-van der Swan D L C, Karssen C M. Theor Appl Genet. 1982;61:385–393. doi: 10.1007/BF00272861. [DOI] [PubMed] [Google Scholar]
- 39.Karssen C M, Brinkhorst-van der Swan D L C, Breekland A E, Koornneef M. Planta. 1983;157:158–165. doi: 10.1007/BF00393650. [DOI] [PubMed] [Google Scholar]
- 40.Rock C D, Heath T G, Gage D A, Zeevaart J A D. Plant Physiol. 1991;97:670–676. doi: 10.1104/pp.97.2.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taylor I B, Tarr A R. Theor Appl Genet. 1984;68:115–119. doi: 10.1007/BF00252325. [DOI] [PubMed] [Google Scholar]
- 42.Groot S P C, Karssen C M. Plant Physiol. 1992;99:952–958. doi: 10.1104/pp.99.3.952. [DOI] [PMC free article] [PubMed] [Google Scholar]