Abstract
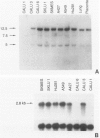
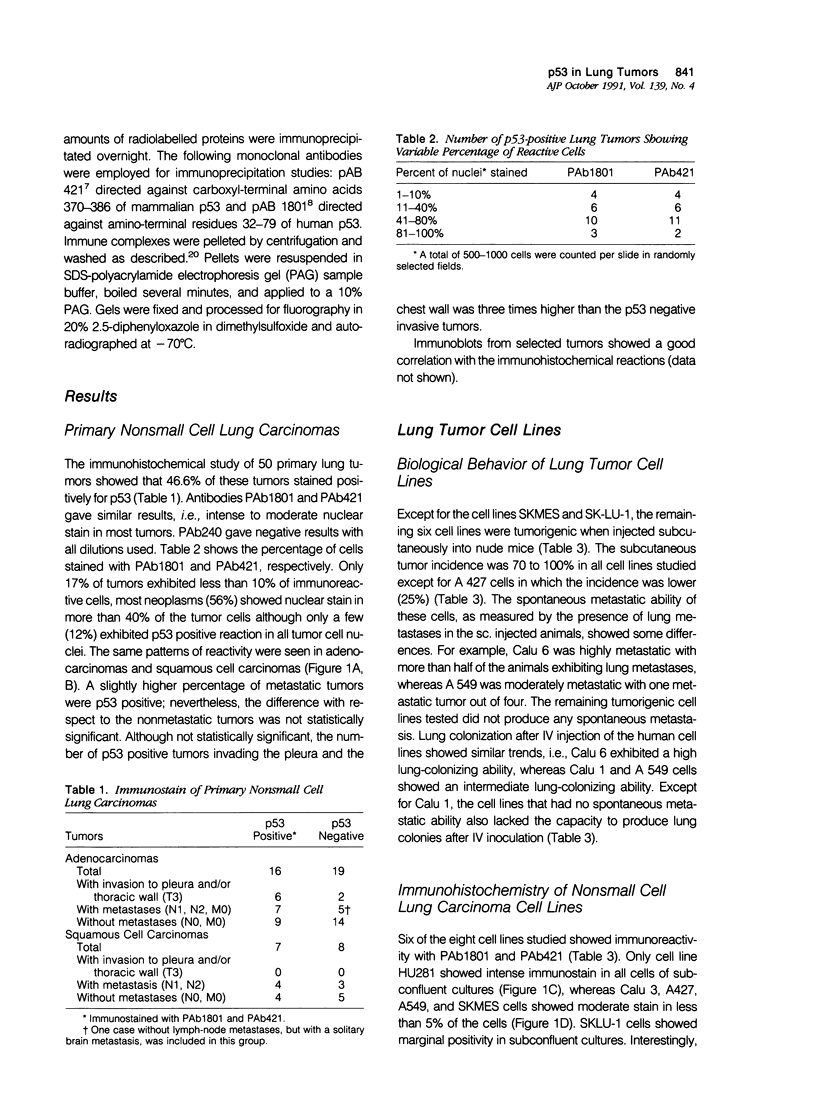
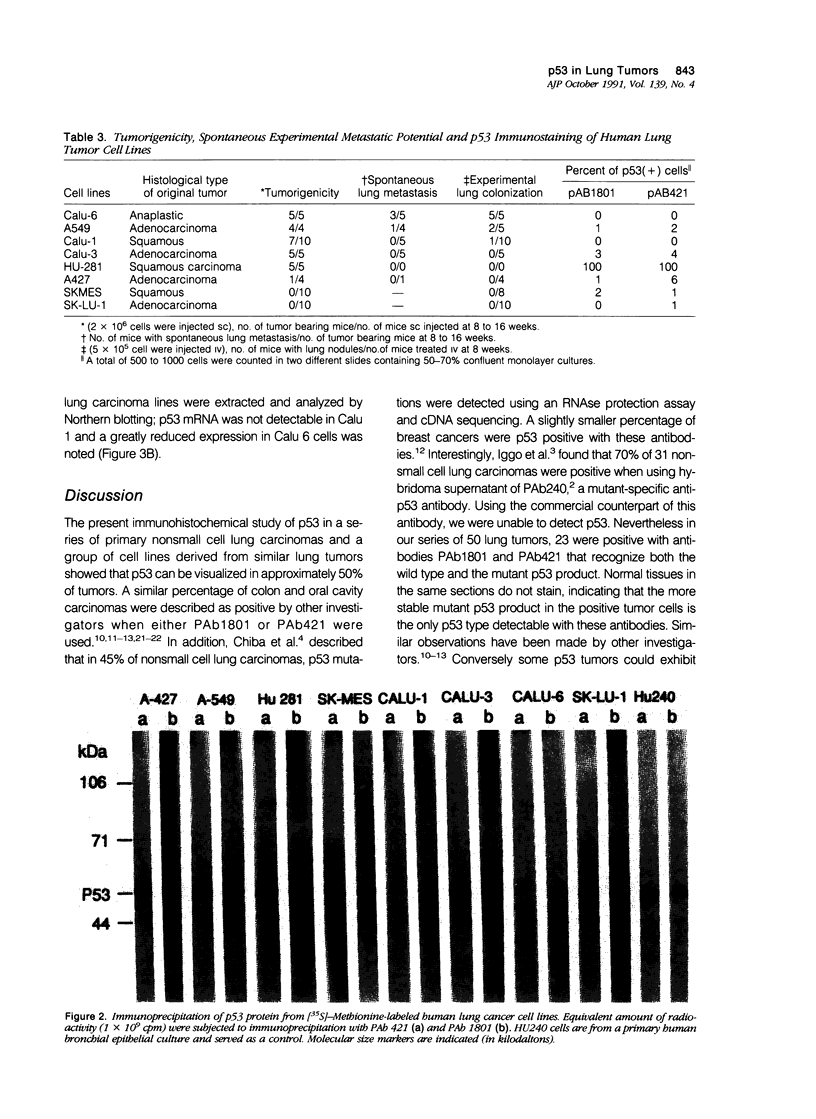
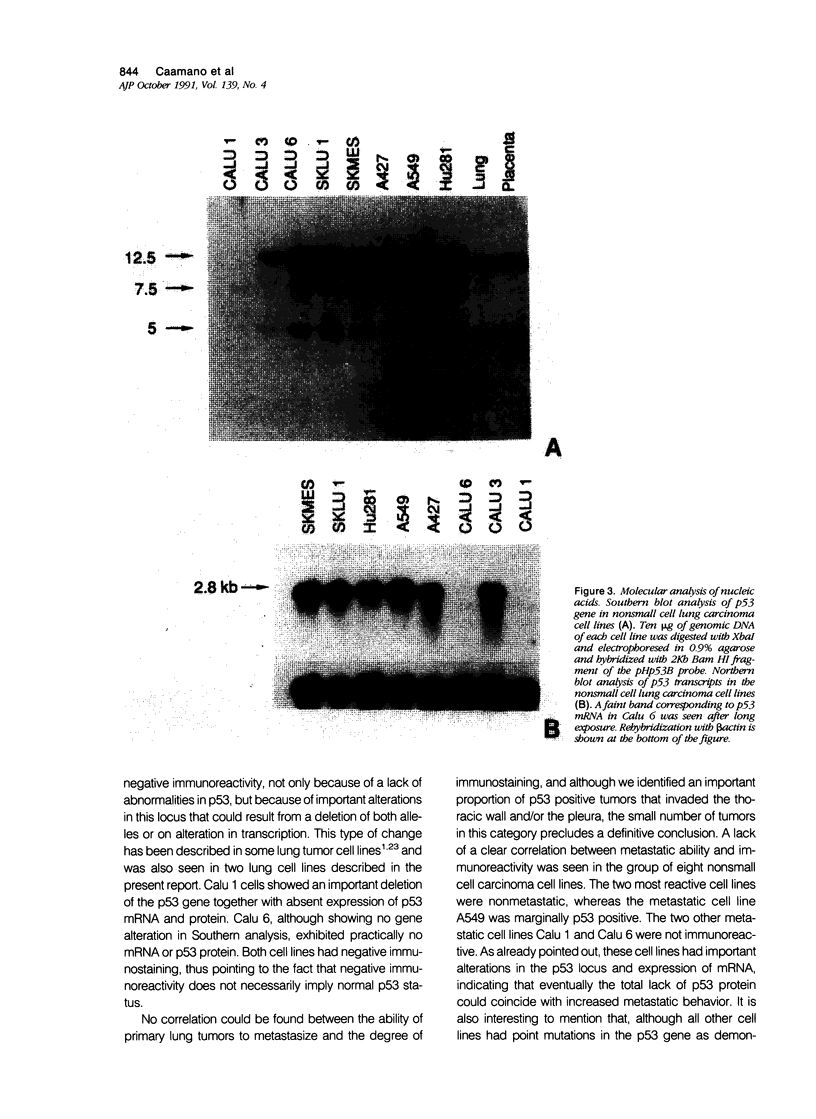
Immunohistochemical analysis of p53, a nuclear protein involved in the development of numerous human tumors, was performed in a series of 50 primary nonsmall cell lung carcinomas and in a group of eight lung carcinoma cell lines. Using two mouse monoclonal antibodies, PAb1801 and PAb421, sixteen of thirty-five (45.7%) lung adenocarcinomas and seven of fifteen (46.6%) squamous cell carcinomas showed marked-to-moderate immunoreactivity. In fifty-six percent of the positive tumors more than 40% of all cells were p53 positive, and in only 17% of positive tumors the percentage of immunostained cells was less than ten. Although the number of p53 negative adenocarcinomas without metastasis was larger than the number of p53 positive tumors without metastasis, there were not clear differences between p53 positive and negative tumors with metastasis. Furthermore, six adenocarcinomas that infiltrated the pleura and/or the thoracic wall were p53 positive, whereas only two of these invasive tumors were p53 negative. From eight cell lines studied, six were positive for p53. A good correlation between immunocytochemistry and immunoprecipitation was observed. Two tumorigenic and metastatic cell lines, Calu 1 and Calu 6, that were not immunoreactive also showed lack of protein by immunoprecipitation, as well as absence of mRNA in Northern analysis. In addition, Calu 1 showed an important gene deletion. These observations point to the fact that deletions and alterations in transcription of the p53 gene could coincide with or eventuate in an advanced malignant phenotype that nevertheless results in a p53 negative immunostain. Although this type of change cannot be detected immunohistochemically in primary tumors without further molecular analysis, the results presented herein indicate that p53 can be detected immunohistochemically in a majority of lung tumors and that there is a tendency for more advanced adenocarcinoma stages to exhibit positive p53 immunostain.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banks L., Matlashewski G., Crawford L. Isolation of human-p53-specific monoclonal antibodies and their use in the studies of human p53 expression. Eur J Biochem. 1986 Sep 15;159(3):529–534. doi: 10.1111/j.1432-1033.1986.tb09919.x. [DOI] [PubMed] [Google Scholar]
- Cattoretti G., Rilke F., Andreola S., D'Amato L., Delia D. P53 expression in breast cancer. Int J Cancer. 1988 Feb 15;41(2):178–183. doi: 10.1002/ijc.2910410204. [DOI] [PubMed] [Google Scholar]
- Chiba I., Takahashi T., Nau M. M., D'Amico D., Curiel D. T., Mitsudomi T., Buchhagen D. L., Carbone D., Piantadosi S., Koga H. Mutations in the p53 gene are frequent in primary, resected non-small cell lung cancer. Lung Cancer Study Group. Oncogene. 1990 Oct;5(10):1603–1610. [PubMed] [Google Scholar]
- Fearon E. R., Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990 Jun 1;61(5):759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Finlay C. A., Hinds P. W., Levine A. J. The p53 proto-oncogene can act as a suppressor of transformation. Cell. 1989 Jun 30;57(7):1083–1093. doi: 10.1016/0092-8674(89)90045-7. [DOI] [PubMed] [Google Scholar]
- Gannon J. V., Greaves R., Iggo R., Lane D. P. Activating mutations in p53 produce a common conformational effect. A monoclonal antibody specific for the mutant form. EMBO J. 1990 May;9(5):1595–1602. doi: 10.1002/j.1460-2075.1990.tb08279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbour J. W., Lai S. L., Whang-Peng J., Gazdar A. F., Minna J. D., Kaye F. J. Abnormalities in structure and expression of the human retinoblastoma gene in SCLC. Science. 1988 Jul 15;241(4863):353–357. doi: 10.1126/science.2838909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E., Crawford L. V., Pim D. C., Williamson N. M. Monoclonal antibodies specific for simian virus 40 tumor antigens. J Virol. 1981 Sep;39(3):861–869. doi: 10.1128/jvi.39.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds P. W., Finlay C. A., Frey A. B., Levine A. J. Immunological evidence for the association of p53 with a heat shock protein, hsc70, in p53-plus-ras-transformed cell lines. Mol Cell Biol. 1987 Aug;7(8):2863–2869. doi: 10.1128/mcb.7.8.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iggo R., Gatter K., Bartek J., Lane D., Harris A. L. Increased expression of mutant forms of p53 oncogene in primary lung cancer. Lancet. 1990 Mar 24;335(8691):675–679. doi: 10.1016/0140-6736(90)90801-b. [DOI] [PubMed] [Google Scholar]
- Lehman T. A., Bennett W. P., Metcalf R. A., Welsh J. A., Ecker J., Modali R. V., Ullrich S., Romano J. W., Appella E., Testa J. R. p53 mutations, ras mutations, and p53-heat shock 70 protein complexes in human lung carcinoma cell lines. Cancer Res. 1991 Aug 1;51(15):4090–4096. [PubMed] [Google Scholar]
- Nigro J. M., Baker S. J., Preisinger A. C., Jessup J. M., Hostetter R., Cleary K., Bigner S. H., Davidson N., Baylin S., Devilee P. Mutations in the p53 gene occur in diverse human tumour types. Nature. 1989 Dec 7;342(6250):705–708. doi: 10.1038/342705a0. [DOI] [PubMed] [Google Scholar]
- Purdie C. A., O'Grady J., Piris J., Wyllie A. H., Bird C. C. p53 expression in colorectal tumors. Am J Pathol. 1991 Apr;138(4):807–813. [PMC free article] [PubMed] [Google Scholar]
- Rodrigues N. R., Rowan A., Smith M. E., Kerr I. B., Bodmer W. F., Gannon J. V., Lane D. P. p53 mutations in colorectal cancer. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7555–7559. doi: 10.1073/pnas.87.19.7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., D'Amico D., Chiba I., Buchhagen D. L., Minna J. D. Identification of intronic point mutations as an alternative mechanism for p53 inactivation in lung cancer. J Clin Invest. 1990 Jul;86(1):363–369. doi: 10.1172/JCI114710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Nau M. M., Chiba I., Birrer M. J., Rosenberg R. K., Vinocour M., Levitt M., Pass H., Gazdar A. F., Minna J. D. p53: a frequent target for genetic abnormalities in lung cancer. Science. 1989 Oct 27;246(4929):491–494. doi: 10.1126/science.2554494. [DOI] [PubMed] [Google Scholar]
- Ura H., Bonfil R. D., Reich R., Reddel R., Pfeifer A., Harris C. C., Klein-Szanto A. J. Expression of type IV collagenase and procollagen genes and its correlation with the tumorigenic, invasive, and metastatic abilities of oncogene-transformed human bronchial epithelial cells. Cancer Res. 1989 Aug 15;49(16):4615–4621. [PubMed] [Google Scholar]
- Zakut-Houri R., Bienz-Tadmor B., Givol D., Oren M. Human p53 cellular tumor antigen: cDNA sequence and expression in COS cells. EMBO J. 1985 May;4(5):1251–1255. doi: 10.1002/j.1460-2075.1985.tb03768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg F. M., Tigges A. J., Schipper M. E., den Hartog-Jager F. C., Kroes W. G., Walboomers J. M. Expression of the nuclear oncogene p53 in colon tumours. J Pathol. 1989 Mar;157(3):193–199. doi: 10.1002/path.1711570304. [DOI] [PubMed] [Google Scholar]