Abstract
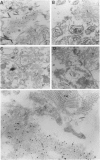
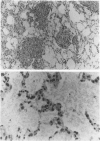
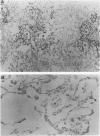
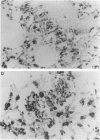
Surfactant protein D (SP-D) (CP4) is a collagenous surfactant-associated carbohydrate binding protein that is synthesized and secreted by alveolar epithelial cells. Previous studies have shown that intratracheal administration of crystalline silica to rats elicits a marked increase in the alveolar accumulation of surfactant lipids and surfactant protein A (SP-A). The authors examined the accumulation of SP-D using this animal model of alveolar proteinosis. Immunoperoxidase localization of SP-D studies at 2 weeks after silica instillation showed intense staining of intra-alveolar exudates, and cytoplasmic staining of hypertrophic type II cells. Immunoelectron microscopy showed that airspace SP-D was specifically associated with granular material, but not tubular myelin or other membranous structures. SP-D was quantified in bronchoalveolar lavage by immunoassay using antibodies specific for SP-D, and by reversephase HPLC after affinity purification of SP-D on maltosyl-agarose. Within 2 weeks after silica instillation, there was a greater than 45-fold increase in lavage SP-D per lung compared with saline controls, including an almost ten-fold increase in the insoluble or surfactant-associated protein. These studies indicate that the extracellular accumulation of SP-D is markedly increased in silica-induced lipoproteinosis, and that SP-D is associated with amorphous components identified by electron microscopy.
Full text
PDF




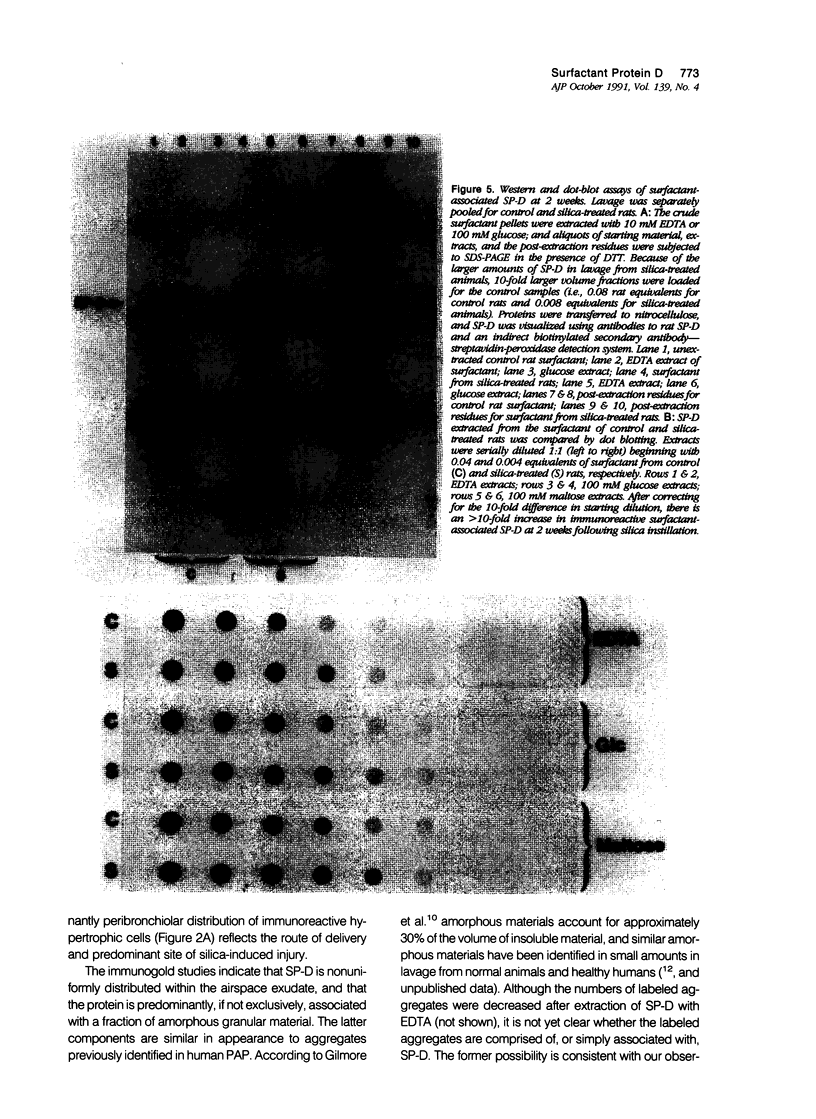
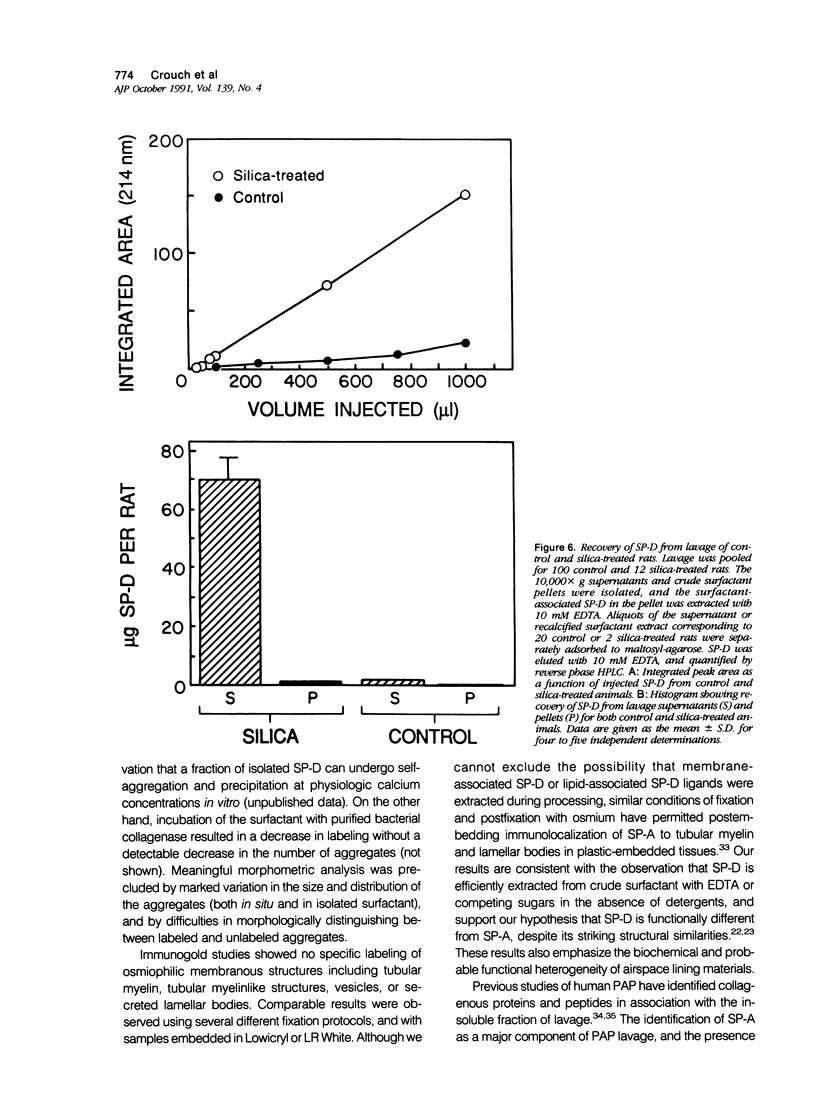


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhattacharyya S. N. Characterization of collagenous and non-collagenous peptides of a glycoprotein isolated from alveoli of patients with alveolar proteinosis. Biochem J. 1981 Feb 1;193(2):447–457. doi: 10.1042/bj1930447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S. N., Lynn W. S. Structural studies on a collagen-like glycoprotein isolated from lung lavage of normal animal. Biochim Biophys Acta. 1980 Dec 16;626(2):451–458. doi: 10.1016/0005-2795(80)90141-5. [DOI] [PubMed] [Google Scholar]
- Buechner H. A., Ansari A. Acute silico-proteinosis. A new pathologic variant of acute silicosis in sandblasters, characterized by histologic features resembling alveolar proteinosis. Dis Chest. 1969 Apr;55(4):274–278. doi: 10.1378/chest.55.4.274. [DOI] [PubMed] [Google Scholar]
- Claypool W. D., Rogers R. M., Matuschak G. M. Update on the clinical diagnosis, management, and pathogenesis of pulmonary alveolar proteinosis (phospholipidosis). Chest. 1984 Apr;85(4):550–558. doi: 10.1378/chest.85.4.550. [DOI] [PubMed] [Google Scholar]
- Coalson J. J., Winter V. T., Martin H. M., King R. J. Colloidal gold immunoultrastructural localization of rat surfactant. Am Rev Respir Dis. 1986 Feb;133(2):230–237. doi: 10.1164/arrd.1986.133.2.230. [DOI] [PubMed] [Google Scholar]
- Crawford S. W., Mecham R. P., Sage H. Structural characteristics and intermolecular organization of human pulmonary-surfactant-associated proteins. Biochem J. 1986 Nov 15;240(1):107–114. doi: 10.1042/bj2400107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouch E., Rust K., Persson A., Mariencheck W., Moxley M., Longmore W. Primary translation products of pulmonary surfactant protein D. Am J Physiol. 1991 Apr;260(4 Pt 1):L247–L253. doi: 10.1152/ajplung.1991.260.4.L247. [DOI] [PubMed] [Google Scholar]
- Dethloff L. A., Gilmore L. B., Brody A. R., Hook G. E. Induction of intra- and extra-cellular phospholipids in the lungs of rats exposed to silica. Biochem J. 1986 Jan 1;233(1):111–118. doi: 10.1042/bj2330111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dethloff L. A., Gladen B. C., Gilmore L. B., Hook G. E. Kinetics of pulmonary surfactant phosphatidylcholine metabolism in the lungs of silica-treated rats. Toxicol Appl Pharmacol. 1989 Mar 15;98(1):1–11. doi: 10.1016/0041-008x(89)90128-2. [DOI] [PubMed] [Google Scholar]
- Gilmore L. B., Talley F. A., Hook G. E. Classification and morphometric quantitation of insoluble materials from the lungs of patients with alveolar proteinosis. Am J Pathol. 1988 Nov;133(2):252–264. [PMC free article] [PubMed] [Google Scholar]
- Golde D. W., Territo M., Finley T. N., Cline M. J. Defective lung macrophages in pulmonary alveolar proteinosis. Ann Intern Med. 1976 Sep;85(3):304–309. doi: 10.7326/0003-4819-85-3-304. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Rothi R. J., Harris J. O. Pulmonary alveolar proteinosis. Further evaluation of abnormal alveolar macrophages. Chest. 1986 Nov;90(5):656–661. doi: 10.1378/chest.90.5.656. [DOI] [PubMed] [Google Scholar]
- Heppleston A. G. Animal model of human disease. Pulmonary alveolar lipo-proteinosis. Animal model: Silica-induced pulmonary alveolar lipo-proteinosis. Am J Pathol. 1975 Jan;78(1):171–174. [PMC free article] [PubMed] [Google Scholar]
- Hoffman R. M., Dauber J. H., Rogers R. M. Improvement in alveolar macrophage migration after therapeutic whole lung lavage in pulmonary alveolar proteinosis. Am Rev Respir Dis. 1989 Apr;139(4):1030–1032. doi: 10.1164/ajrccm/139.4.1030. [DOI] [PubMed] [Google Scholar]
- Hook G. E., Bell D. Y., Gilmore L. B., Nadeau D., Reasor M. J., Talley F. A. Composition of bronchoalveolar lavage effluents from patients with pulmonary alveolar proteinosis. Lab Invest. 1978 Oct;39(4):342–357. [PubMed] [Google Scholar]
- Hook G. E., Gilmore L. B., Talley F. A. Dissolution and reassembly of tubular myelin-like multilamellated structures from the lungs of patients with pulmonary alveolar proteinosis. Lab Invest. 1986 Aug;55(2):194–208. [PubMed] [Google Scholar]
- Kawada H., Horiuchi T., Shannon J. M., Kuroki Y., Voelker D. R., Mason R. J. Alveolar type II cells, surfactant protein A (SP-A), and the phospholipid components of surfactant in acute silicosis in the rat. Am Rev Respir Dis. 1989 Aug;140(2):460–470. doi: 10.1164/ajrccm/140.2.460. [DOI] [PubMed] [Google Scholar]
- Kuhn C., 3rd, Boldt J., King T. E., Jr, Crouch E., Vartio T., McDonald J. A. An immunohistochemical study of architectural remodeling and connective tissue synthesis in pulmonary fibrosis. Am Rev Respir Dis. 1989 Dec;140(6):1693–1703. doi: 10.1164/ajrccm/140.6.1693. [DOI] [PubMed] [Google Scholar]
- McDonald J. A., Broekelmann T. J., Matheke M. L., Crouch E., Koo M., Kuhn C., 3rd A monoclonal antibody to the carboxyterminal domain of procollagen type I visualizes collagen-synthesizing fibroblasts. Detection of an altered fibroblast phenotype in lungs of patients with pulmonary fibrosis. J Clin Invest. 1986 Nov;78(5):1237–1244. doi: 10.1172/JCI112707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEuen D. D., Abraham J. L. Particulate concentrations in pulmonary alveolar proteinosis. Environ Res. 1978 Dec;17(3):334–339. doi: 10.1016/0013-9351(78)90037-3. [DOI] [PubMed] [Google Scholar]
- Miller B. E., Bakewell W. E., Katyal S. L., Singh G., Hook G. E. Induction of surfactant protein (SP-A) biosynthesis and SP-A mRNA in activated type II cells during acute silicosis in rats. Am J Respir Cell Mol Biol. 1990 Sep;3(3):217–226. doi: 10.1165/ajrcmb/3.3.217. [DOI] [PubMed] [Google Scholar]
- Miller B. E., Dethloff L. A., Gladen B. C., Hook G. E. Progression of type II cell hypertrophy and hyperplasia during silica-induced pulmonary inflammation. Lab Invest. 1987 Nov;57(5):546–554. [PubMed] [Google Scholar]
- Miller B. E., Dethloff L. A., Hook G. E. Silica-induced hypertrophy of type II cells in the lungs of rats. Lab Invest. 1986 Aug;55(2):153–163. [PubMed] [Google Scholar]
- Miller B. E., Hook G. E. Stimulation of surfactant phospholipid biosynthesis in the lungs of rats treated with silica. Biochem J. 1988 Aug 1;253(3):659–665. doi: 10.1042/bj2530659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. R., Churg A. M., Hutcheon M., Lom S. Pulmonary alveolar proteinosis and aluminum dust exposure. Am Rev Respir Dis. 1984 Aug;130(2):312–315. doi: 10.1164/arrd.1984.130.2.312. [DOI] [PubMed] [Google Scholar]
- Panos R. J., Suwabe A., Leslie C. C., Mason R. J. Hypertrophic alveolar type II cells from silica-treated rats are committed to DNA synthesis in vitro. Am J Respir Cell Mol Biol. 1990 Jul;3(1):51–59. doi: 10.1165/ajrcmb/3.1.51. [DOI] [PubMed] [Google Scholar]
- Persson A., Chang D., Crouch E. Surfactant protein D is a divalent cation-dependent carbohydrate-binding protein. J Biol Chem. 1990 Apr 5;265(10):5755–5760. [PubMed] [Google Scholar]
- Persson A., Chang D., Rust K., Moxley M., Longmore W., Crouch E. Purification and biochemical characterization of CP4 (SP-D), a collagenous surfactant-associated protein. Biochemistry. 1989 Jul 25;28(15):6361–6367. doi: 10.1021/bi00441a031. [DOI] [PubMed] [Google Scholar]
- Persson A., Rust K., Chang D., Moxley M., Longmore W., Crouch E. CP4: a pneumocyte-derived collagenous surfactant-associated protein. Evidence for heterogeneity of collagenous surfactant proteins. Biochemistry. 1988 Nov 15;27(23):8576–8584. doi: 10.1021/bi00423a011. [DOI] [PubMed] [Google Scholar]
- Phelps D. S., Taeusch H. W., Jr, Benson B., Hawgood S. An electrophoretic and immunochemical characterization of human surfactant-associated proteins. Biochim Biophys Acta. 1984 Dec 7;791(2):226–238. doi: 10.1016/0167-4838(84)90013-x. [DOI] [PubMed] [Google Scholar]
- ROSEN S. H., CASTLEMAN B., LIEBOW A. A. Pulmonary alveolar proteinosis. N Engl J Med. 1958 Jun 5;258(23):1123–1142. doi: 10.1056/NEJM195806052582301. [DOI] [PubMed] [Google Scholar]
- Ross G. F., Ohning B. L., Tannenbaum D., Whitsett J. A. Structural relationships of the major glycoproteins from human alveolar proteinosis surfactant. Biochim Biophys Acta. 1987 Feb 25;911(3):294–305. doi: 10.1016/0167-4838(87)90070-7. [DOI] [PubMed] [Google Scholar]
- Rubin R. W., Warren R. W. Quantitation of microgram amounts of protein in SDS-mercaptoethanol-tris electrophoresis sample buffer. Anal Biochem. 1977 Dec;83(2):773–777. doi: 10.1016/0003-2697(77)90084-7. [DOI] [PubMed] [Google Scholar]
- Singh G., Katyal S. L., Bedrossian C. W., Rogers R. M. Pulmonary alveolar proteinosis. Staining for surfactant apoprotein in alveolar proteinosis and in conditions simulating it. Chest. 1983 Jan;83(1):82–86. doi: 10.1378/chest.83.1.82. [DOI] [PubMed] [Google Scholar]
- Takemura T., Fukuda Y., Harrison M., Ferrans V. J. Ultrastructural, histochemical, and freeze-fracture evaluation of multilamellated structures in human pulmonary alveolar proteinosis. Am J Anat. 1987 Jul;179(3):258–268. doi: 10.1002/aja.1001790307. [DOI] [PubMed] [Google Scholar]
- White R. T., Damm D., Miller J., Spratt K., Schilling J., Hawgood S., Benson B., Cordell B. Isolation and characterization of the human pulmonary surfactant apoprotein gene. 1985 Sep 26-Oct 2Nature. 317(6035):361–363. doi: 10.1038/317361a0. [DOI] [PubMed] [Google Scholar]
- Whitsett J. A., Hull W., Ross G., Weaver T. Characteristics of human surfactant-associated glycoproteins A. Pediatr Res. 1985 May;19(5):501–508. doi: 10.1203/00006450-198505000-00018. [DOI] [PubMed] [Google Scholar]