Abstract
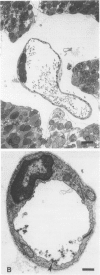
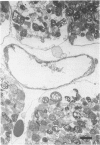
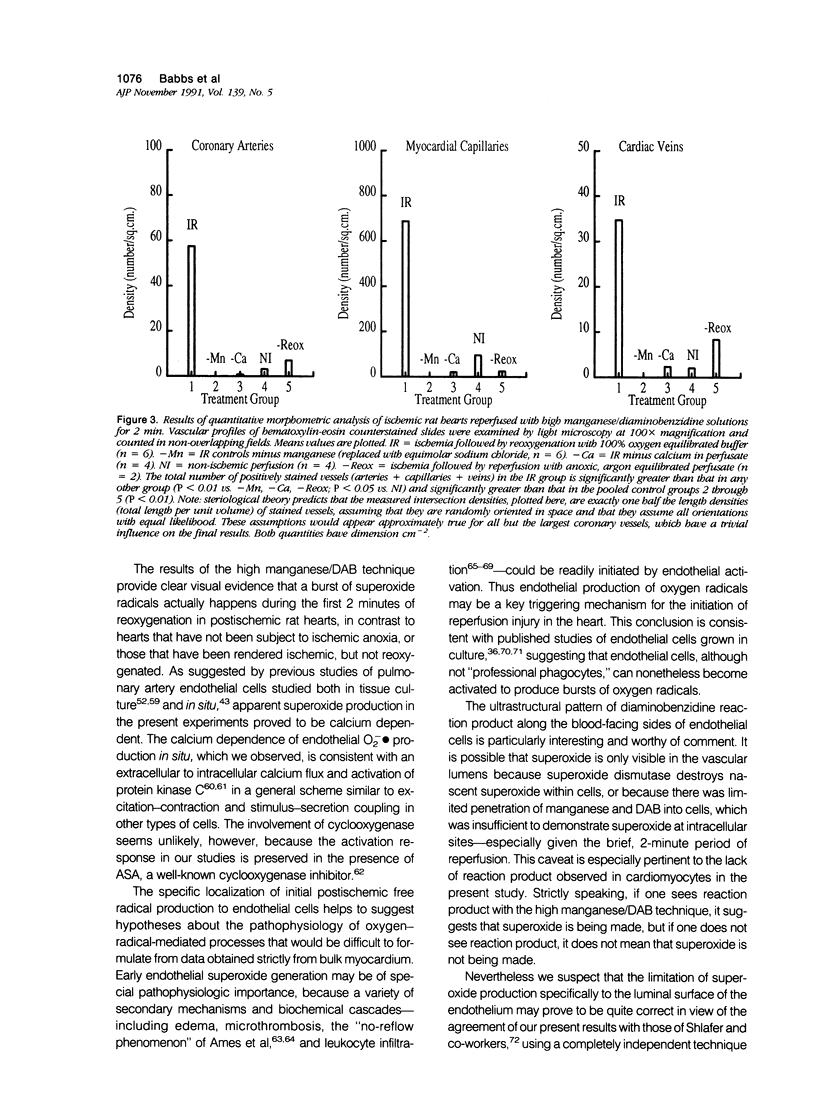
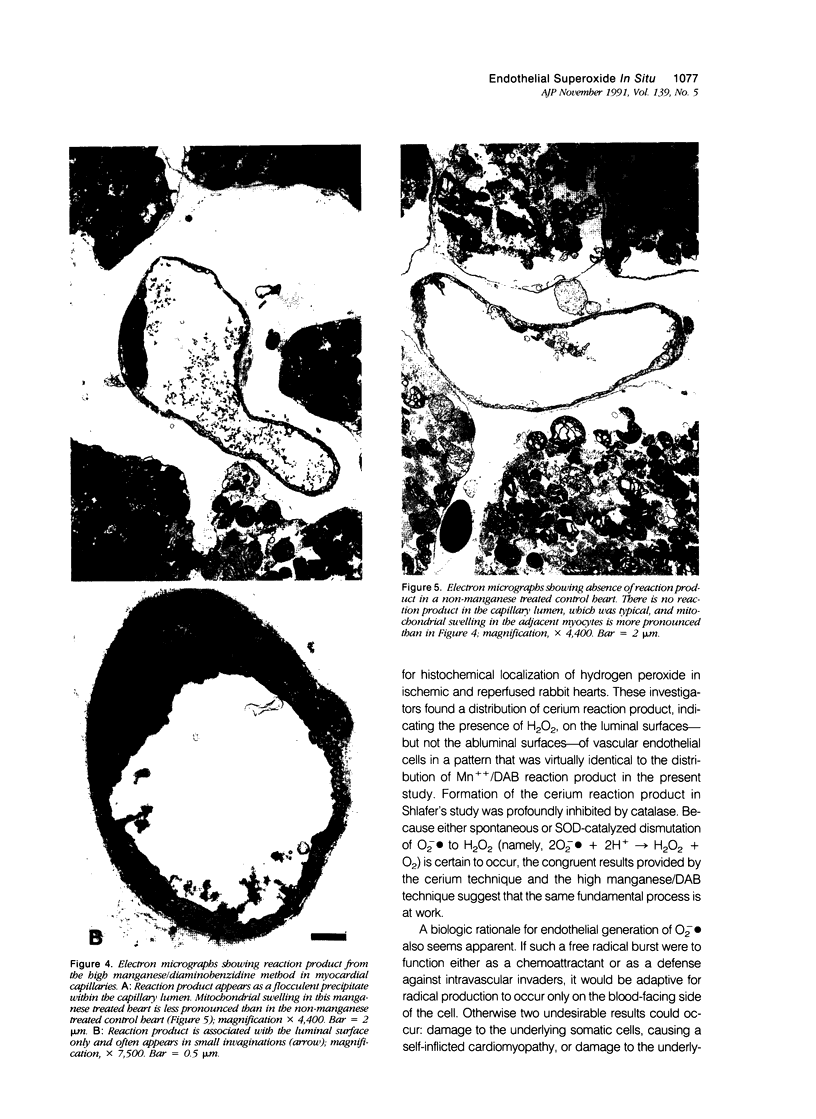
This paper describes a histochemical study of superoxide generation in buffer-perfused, isolated rat hearts during the first 2 minutes of reperfusion after 60 minutes of warm ischemia. Superoxide radical production was demonstrated by a modification of Karnovsky's manganese/diaminobenzidine technique, in which superoxide oxidizes Mn++ to Mn ions, which in turn oxidize diaminobenzidine to form amber, osmiophilic polymers, observable by light or electron microscopy. Isolated hearts were rendered ischemic, reperfused with oxygen equilibrated buffer containing Mn++ and diaminobenzidine, fixed by perfusion with Trump's solution, and processed for light and electron microscopy. The method consistently demonstrated evidence of superoxide generation near the luminal surfaces of arterial, capillary, and venular endothelial cells during the first 2 minutes of reoxygenation after ischemia. The histochemical reaction was absent or markedly reduced in non-manganese-treated or nonischemic hearts, as well as in hearts perfused with calcium-free or oxygen-free buffers. The histochemical differences were statistically significant on quantitative morphometric analysis. These results provide direct, visual evidence of the existence and endothelial localization of a burst of superoxide radicals in intact, postischemic myocardium and suggest the pathophysiologic importance of calcium-dependent endothelial cell activation in the initiation of reperfusion injury.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akizuki S., Yoshida S., Chambers D. E., Eddy L. J., Parmley L. F., Yellon D. M., Downey J. M. Infarct size limitation by the xanthine oxidase inhibitor, allopurinol, in closed-chest dogs with small infarcts. Cardiovasc Res. 1985 Nov;19(11):686–692. doi: 10.1093/cvr/19.11.686. [DOI] [PubMed] [Google Scholar]
- Ambrosio G., Zweier J. L., Jacobus W. E., Weisfeldt M. L., Flaherty J. T. Improvement of postischemic myocardial function and metabolism induced by administration of deferoxamine at the time of reflow: the role of iron in the pathogenesis of reperfusion injury. Circulation. 1987 Oct;76(4):906–915. doi: 10.1161/01.cir.76.4.906. [DOI] [PubMed] [Google Scholar]
- Ames A., 3rd, Wright R. L., Kowada M., Thurston J. M., Majno G. Cerebral ischemia. II. The no-reflow phenomenon. Am J Pathol. 1968 Feb;52(2):437–453. [PMC free article] [PubMed] [Google Scholar]
- Archibald F. S., Fridovich I. The scavenging of superoxide radical by manganous complexes: in vitro. Arch Biochem Biophys. 1982 Apr 1;214(2):452–463. doi: 10.1016/0003-9861(82)90049-2. [DOI] [PubMed] [Google Scholar]
- Arroyo C. M., Kramer J. H., Dickens B. F., Weglicki W. B. Identification of free radicals in myocardial ischemia/reperfusion by spin trapping with nitrone DMPO. FEBS Lett. 1987 Aug 31;221(1):101–104. doi: 10.1016/0014-5793(87)80360-5. [DOI] [PubMed] [Google Scholar]
- Aust S. D., Morehouse L. A., Thomas C. E. Role of metals in oxygen radical reactions. J Free Radic Biol Med. 1985;1(1):3–25. doi: 10.1016/0748-5514(85)90025-x. [DOI] [PubMed] [Google Scholar]
- Aznar J., Santos M. T., Valles J., Sala J. Serum malondialdehyde-like material (MDA-LM) in acute myocardial infarction. J Clin Pathol. 1983 Jun;36(6):712–715. doi: 10.1136/jcp.36.6.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babbs C. F. Reperfusion injury of postischemic tissues. Ann Emerg Med. 1988 Nov;17(11):1148–1157. doi: 10.1016/s0196-0644(88)80060-x. [DOI] [PubMed] [Google Scholar]
- Babbs C. F. Role of iron ions in the genesis of reperfusion injury following successful cardiopulmonary resuscitation: preliminary data and a biochemical hypothesis. Ann Emerg Med. 1985 Aug;14(8):777–783. doi: 10.1016/s0196-0644(85)80056-1. [DOI] [PubMed] [Google Scholar]
- Babbs C. F., Salaris S. C., Turek J. J. Cytochemical studies of hydrogen peroxide generation in postischemic hepatocytes. Am J Physiol. 1991 Jan;260(1 Pt 2):H123–H129. doi: 10.1152/ajpheart.1991.260.1.H123. [DOI] [PubMed] [Google Scholar]
- Badylak S. F., Simmons A., Turek J., Babbs C. F. Protection from reperfusion injury in the isolated rat heart by postischaemic deferoxamine and oxypurinol administration. Cardiovasc Res. 1987 Jul;21(7):500–506. doi: 10.1093/cvr/21.7.500. [DOI] [PubMed] [Google Scholar]
- Baker J. E., Felix C. C., Olinger G. N., Kalyanaraman B. Myocardial ischemia and reperfusion: direct evidence for free radical generation by electron spin resonance spectroscopy. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2786–2789. doi: 10.1073/pnas.85.8.2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battelli M. G., Corte E. D., Stirpe F. Xanthine oxidase type D (dehydrogenase) in the intestine and other organs of the rat. Biochem J. 1972 Feb;126(3):747–749. doi: 10.1042/bj1260747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs R. T., Robinson J. M., Karnovsky M. L., Karnovsky M. J. Superoxide production by polymorphonuclear leukocytes. A cytochemical approach. Histochemistry. 1986;84(4-6):371–378. doi: 10.1007/BF00482965. [DOI] [PubMed] [Google Scholar]
- Burney R. E., Walsh D., Kaplan L. R., Fraser S., Tung B., Overmyer J. Reperfusion arrhythmia: myth or reality? Ann Emerg Med. 1989 Mar;18(3):240–243. doi: 10.1016/s0196-0644(89)80404-4. [DOI] [PubMed] [Google Scholar]
- Chambers D. E., Parks D. A., Patterson G., Roy R., McCord J. M., Yoshida S., Parmley L. F., Downey J. M. Xanthine oxidase as a source of free radical damage in myocardial ischemia. J Mol Cell Cardiol. 1985 Feb;17(2):145–152. doi: 10.1016/s0022-2828(85)80017-1. [DOI] [PubMed] [Google Scholar]
- Eddy L. J., Stewart J. R., Jones H. P., Engerson T. D., McCord J. M., Downey J. M. Free radical-producing enzyme, xanthine oxidase, is undetectable in human hearts. Am J Physiol. 1987 Sep;253(3 Pt 2):H709–H711. doi: 10.1152/ajpheart.1987.253.3.H709. [DOI] [PubMed] [Google Scholar]
- Elias H., Hennig A., Schwartz D. E. Stereology: applications to biomedicalresearch. Physiol Rev. 1971 Jan;51(1):158–200. doi: 10.1152/physrev.1971.51.1.158. [DOI] [PubMed] [Google Scholar]
- Fahimi H. D. Cytochemical localization of peroxidase activity in rat hepatic microbodies (peroxisomes). J Histochem Cytochem. 1968 Aug;16(8):547–550. doi: 10.1177/16.8.547. [DOI] [PubMed] [Google Scholar]
- Farber J. L., Kyle M. E., Coleman J. B. Mechanisms of cell injury by activated oxygen species. Lab Invest. 1990 Jun;62(6):670–679. [PubMed] [Google Scholar]
- Ferrari R., Ceconi C., Curello S., Guarnieri C., Caldarera C. M., Albertini A., Visioli O. Oxygen-mediated myocardial damage during ischaemia and reperfusion: role of the cellular defences against oxygen toxicity. J Mol Cell Cardiol. 1985 Oct;17(10):937–945. doi: 10.1016/s0022-2828(85)80074-2. [DOI] [PubMed] [Google Scholar]
- Freeman B. A., Crapo J. D. Biology of disease: free radicals and tissue injury. Lab Invest. 1982 Nov;47(5):412–426. [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. Annu Rev Biochem. 1975;44:147–159. doi: 10.1146/annurev.bi.44.070175.001051. [DOI] [PubMed] [Google Scholar]
- Gallagher K. P., Buda A. J., Pace D., Gerren R. A., Shlafer M. Failure of superoxide dismutase and catalase to alter size of infarction in conscious dogs after 3 hours of occlusion followed by reperfusion. Circulation. 1986 May;73(5):1065–1076. doi: 10.1161/01.cir.73.5.1065. [DOI] [PubMed] [Google Scholar]
- Garlick P. B., Davies M. J., Hearse D. J., Slater T. F. Direct detection of free radicals in the reperfused rat heart using electron spin resonance spectroscopy. Circ Res. 1987 Nov;61(5):757–760. doi: 10.1161/01.res.61.5.757. [DOI] [PubMed] [Google Scholar]
- Gauduel Y., Duvelleroy M. A. Role of oxygen radicals in cardiac injury due to reoxygenation. J Mol Cell Cardiol. 1984 May;16(5):459–470. doi: 10.1016/s0022-2828(84)80617-3. [DOI] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Guarnieri C., Flamigni F., Caldarera C. M. Role of oxygen in the cellular damage induced by re-oxygenation of hypoxic heart. J Mol Cell Cardiol. 1980 Aug;12(8):797–808. doi: 10.1016/0022-2828(80)90081-4. [DOI] [PubMed] [Google Scholar]
- Hammond B., Hess M. L. The oxygen free radical system: potential mediator of myocardial injury. J Am Coll Cardiol. 1985 Jul;6(1):215–220. doi: 10.1016/s0735-1097(85)80278-3. [DOI] [PubMed] [Google Scholar]
- Hearse D. J., Humphrey S. M., Chain E. B. Abrupt reoxygenation of the anoxic potassium-arrested perfused rat heart: a study of myocardial enzyme release. J Mol Cell Cardiol. 1973 Aug;5(4):395–407. doi: 10.1016/0022-2828(73)90030-8. [DOI] [PubMed] [Google Scholar]
- Herman I. M. Extracellular matrix-cytoskeletal interactions in vascular cells. Tissue Cell. 1987;19(1):1–19. doi: 10.1016/0040-8166(87)90052-8. [DOI] [PubMed] [Google Scholar]
- Hernandez L. A., Grisham M. B., Twohig B., Arfors K. E., Harlan J. M., Granger D. N. Role of neutrophils in ischemia-reperfusion-induced microvascular injury. Am J Physiol. 1987 Sep;253(3 Pt 2):H699–H703. doi: 10.1152/ajpheart.1987.253.3.H699. [DOI] [PubMed] [Google Scholar]
- Hess M. L., Manson N. H. Molecular oxygen: friend and foe. The role of the oxygen free radical system in the calcium paradox, the oxygen paradox and ischemia/reperfusion injury. J Mol Cell Cardiol. 1984 Nov;16(11):969–985. doi: 10.1016/s0022-2828(84)80011-5. [DOI] [PubMed] [Google Scholar]
- Jarasch E. D., Grund C., Bruder G., Heid H. W., Keenan T. W., Franke W. W. Localization of xanthine oxidase in mammary-gland epithelium and capillary endothelium. Cell. 1981 Jul;25(1):67–82. doi: 10.1016/0092-8674(81)90232-4. [DOI] [PubMed] [Google Scholar]
- Johnston W. H., Latta H. Glomerular mesangial and endothelial cell swelling following temporary renal ischemia and its role in the no-reflow phenomenon. Am J Pathol. 1977 Oct;89(1):153–166. [PMC free article] [PubMed] [Google Scholar]
- Jolly S. R., Kane W. J., Bailie M. B., Abrams G. D., Lucchesi B. R. Canine myocardial reperfusion injury. Its reduction by the combined administration of superoxide dismutase and catalase. Circ Res. 1984 Mar;54(3):277–285. doi: 10.1161/01.res.54.3.277. [DOI] [PubMed] [Google Scholar]
- Karnovsky M. J., Robinson J. M., Briggs R. T., Karnovsky M. L. Oxidative cytochemistry in phagocytosis: the interface between structure and function. Histochem J. 1981 Jan;13(1):1–22. doi: 10.1007/BF01005835. [DOI] [PubMed] [Google Scholar]
- Kono Y., Takahashi M. A., Asada K. Oxidation of manganous pyrophosphate by superoxide radicals and illuminated spinach chloroplasts. Arch Biochem Biophys. 1976 Jun;174(2):454–462. doi: 10.1016/0003-9861(76)90373-8. [DOI] [PubMed] [Google Scholar]
- Lenz M. L., Michael L. H., Smith C. V., Hughes H., Shappell S. B., Taylor A. A., Entman M. L., Mitchell J. R. Glutathione disulfide formation and lipid peroxidation during cardiac ischemia and reflow in the dog in vivo. Biochem Biophys Res Commun. 1989 Oct 31;164(2):722–727. doi: 10.1016/0006-291x(89)91519-2. [DOI] [PubMed] [Google Scholar]
- Litwin J. A. Transition metal-catalysed oxidation of 3,3'-diaminobenzidine [DAB] in a model system. Acta Histochem. 1982;71(1):111–117. doi: 10.1016/S0065-1281(82)80023-8. [DOI] [PubMed] [Google Scholar]
- Matsubara T., Ziff M. Superoxide anion release by human endothelial cells: synergism between a phorbol ester and a calcium ionophore. J Cell Physiol. 1986 May;127(2):207–210. doi: 10.1002/jcp.1041270203. [DOI] [PubMed] [Google Scholar]
- McCord J. M. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985 Jan 17;312(3):159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- McCord J. M. Oxygen-derived radicals: a link between reperfusion injury and inflammation. Fed Proc. 1987 May 15;46(7):2402–2406. [PubMed] [Google Scholar]
- Meerson F. Z., Kagan V. E., Kozlov YuP, Belkina L. M., Arkhipenko YuV The role of lipid peroxidation in pathogenesis of ischemic damage and the antioxidant protection of the heart. Basic Res Cardiol. 1982 Sep-Oct;77(5):465–485. doi: 10.1007/BF01907940. [DOI] [PubMed] [Google Scholar]
- Misra H. P., Fridovich I. Inhibition of superoxide dismutases by azide. Arch Biochem Biophys. 1978 Aug;189(2):317–322. doi: 10.1016/0003-9861(78)90218-7. [DOI] [PubMed] [Google Scholar]
- Morrell J. I., Greenberger L. M., Pfaff D. W. Comparison of horseradish peroxidase visualization methods: quantitative results and further technical specifics. J Histochem Cytochem. 1981 Aug;29(8):903–916. doi: 10.1177/29.8.7276535. [DOI] [PubMed] [Google Scholar]
- Myers C. L., Weiss S. J., Kirsh M. M., Shlafer M. Involvement of hydrogen peroxide and hydroxyl radical in the 'oxygen paradox': reduction of creatine kinase release by catalase, allopurinol or deferoxamine, but not by superoxide dismutase. J Mol Cell Cardiol. 1985 Jul;17(7):675–684. doi: 10.1016/s0022-2828(85)80067-5. [DOI] [PubMed] [Google Scholar]
- Nayler W. G., Elz J. S. Reperfusion injury: laboratory artifact or clinical dilemma? Circulation. 1986 Aug;74(2):215–221. doi: 10.1161/01.cir.74.2.215. [DOI] [PubMed] [Google Scholar]
- Nohl H. A novel superoxide radical generator in heart mitochondria. FEBS Lett. 1987 Apr 20;214(2):269–273. doi: 10.1016/0014-5793(87)80068-6. [DOI] [PubMed] [Google Scholar]
- Parks D. A., Granger D. N. Xanthine oxidase: biochemistry, distribution and physiology. Acta Physiol Scand Suppl. 1986;548:87–99. [PubMed] [Google Scholar]
- Przyklenk K., Kloner R. A. Superoxide dismutase plus catalase improve contractile function in the canine model of the "stunned myocardium". Circ Res. 1986 Jan;58(1):148–156. doi: 10.1161/01.res.58.1.148. [DOI] [PubMed] [Google Scholar]
- Rinaldo J. E., Basford R. E. Neutrophil-endothelial interactions: modulation of neutrophil activation responses by endothelial cells. Tissue Cell. 1987;19(5):99–606. [PubMed] [Google Scholar]
- Robinson J. M., Badwey J. A., Karnovsky M. L., Karnovsky M. J. Superoxide release by neutrophils: synergistic effects of a phorbol ester and a calcium ionophore. Biochem Biophys Res Commun. 1984 Jul 31;122(2):734–739. doi: 10.1016/s0006-291x(84)80095-9. [DOI] [PubMed] [Google Scholar]
- Romson J. L., Hook B. G., Kunkel S. L., Abrams G. D., Schork M. A., Lucchesi B. R. Reduction of the extent of ischemic myocardial injury by neutrophil depletion in the dog. Circulation. 1983 May;67(5):1016–1023. doi: 10.1161/01.cir.67.5.1016. [DOI] [PubMed] [Google Scholar]
- Rosen G. M., Freeman B. A. Detection of superoxide generated by endothelial cells. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7269–7273. doi: 10.1073/pnas.81.23.7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligman A. M., Karnovsky M. J., Wasserkrug H. L., Hanker J. S. Nondroplet ultrastructural demonstration of cytochrome oxidase activity with a polymerizing osmiophilic reagent, diaminobenzidine (DAB). J Cell Biol. 1968 Jul;38(1):1–14. doi: 10.1083/jcb.38.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlafer M., Brosamer K., Forder J. R., Simon R. H., Ward P. A., Grum C. M. Cerium chloride as a histochemical marker of hydrogen peroxide in reperfused ischemic hearts. J Mol Cell Cardiol. 1990 Jan;22(1):83–97. doi: 10.1016/0022-2828(90)90974-7. [DOI] [PubMed] [Google Scholar]
- Shlafer M., Kane P. F., Kirsh M. M. Superoxide dismutase plus catalase enhances the efficacy of hypothermic cardioplegia to protect the globally ischemic, reperfused heart. J Thorac Cardiovasc Surg. 1982 Jun;83(6):830–839. [PubMed] [Google Scholar]
- Turrens J. F., Boveris A. Generation of superoxide anion by the NADH dehydrogenase of bovine heart mitochondria. Biochem J. 1980 Nov 1;191(2):421–427. doi: 10.1042/bj1910421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P. A., Johnson K. J., Till G. O. Animal models of oxidant lung injury. Respiration. 1986;50 (Suppl 1):5–12. doi: 10.1159/000195082. [DOI] [PubMed] [Google Scholar]
- Werns S. W., Shea M. J., Mitsos S. E., Dysko R. C., Fantone J. C., Schork M. A., Abrams G. D., Pitt B., Lucchesi B. R. Reduction of the size of infarction by allopurinol in the ischemic-reperfused canine heart. Circulation. 1986 Mar;73(3):518–524. doi: 10.1161/01.cir.73.3.518. [DOI] [PubMed] [Google Scholar]
- Zweier J. L., Flaherty J. T., Weisfeldt M. L. Direct measurement of free radical generation following reperfusion of ischemic myocardium. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1404–1407. doi: 10.1073/pnas.84.5.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweier J. L., Kuppusamy P., Lutty G. A. Measurement of endothelial cell free radical generation: evidence for a central mechanism of free radical injury in postischemic tissues. Proc Natl Acad Sci U S A. 1988 Jun;85(11):4046–4050. doi: 10.1073/pnas.85.11.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]