Abstract
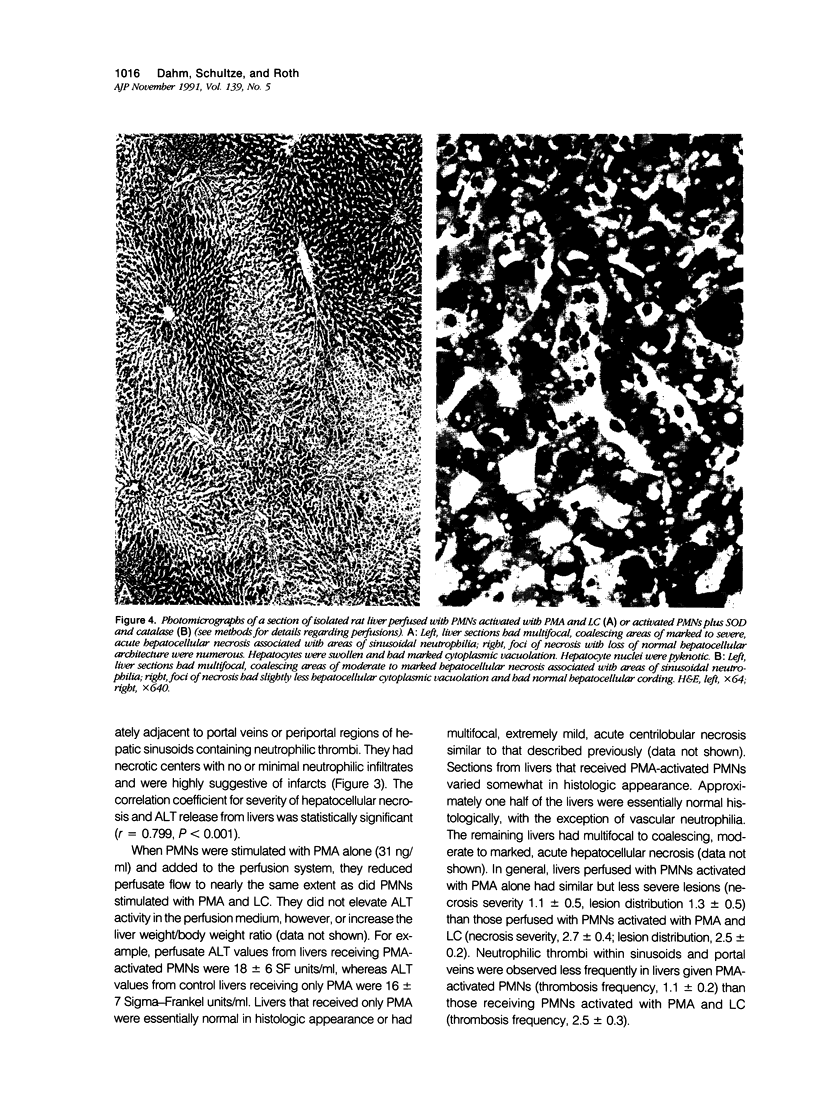
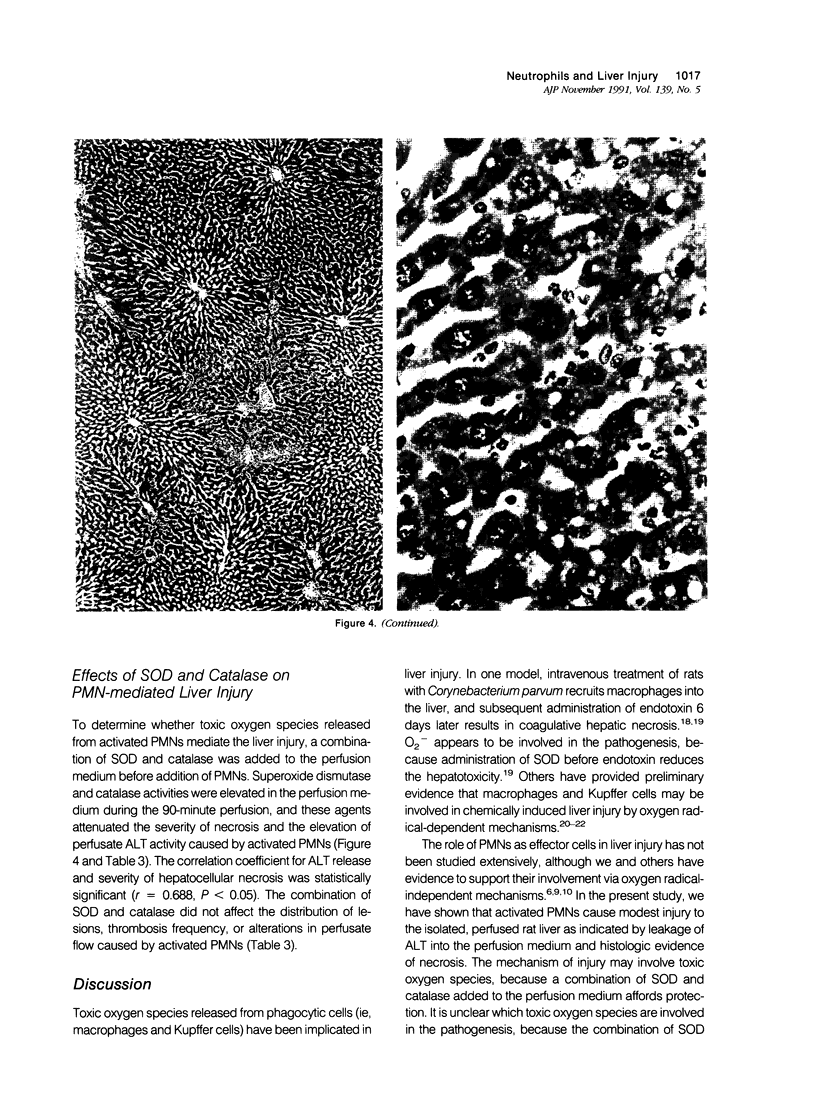
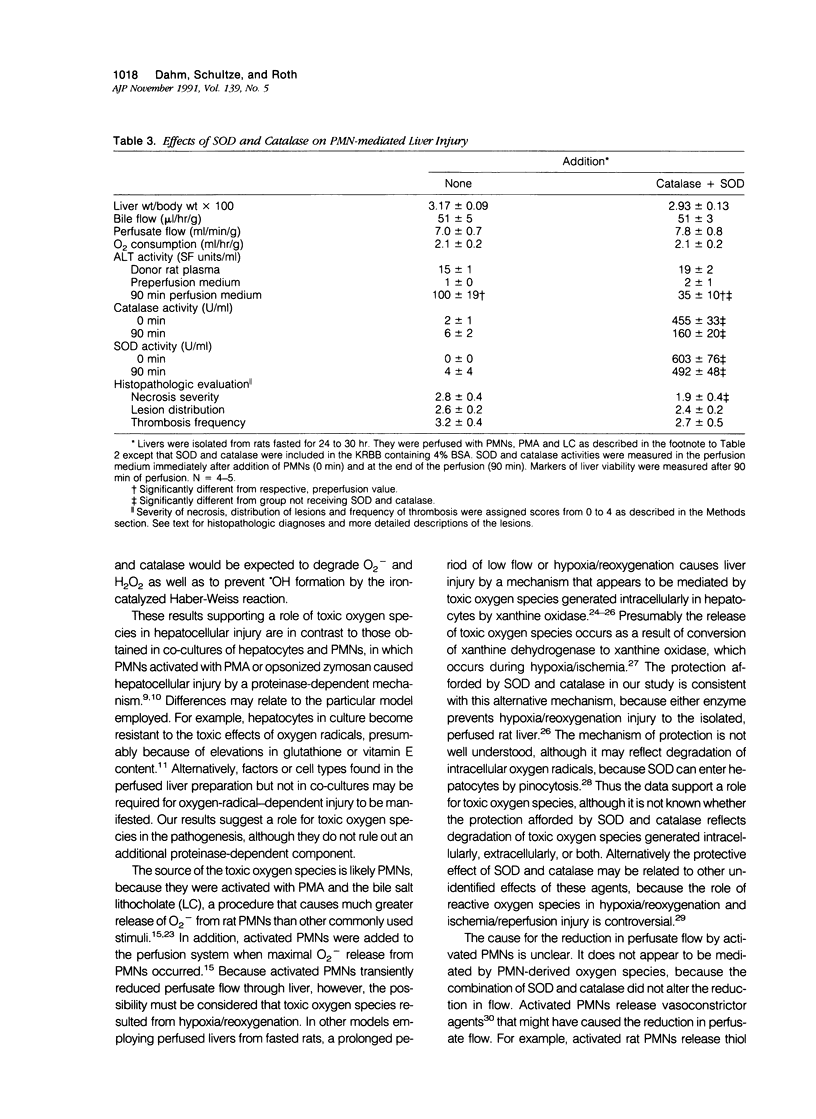
Under certain circumstances, segmented neutrophils (PMNs) injure extrahepatic tissue by releasing toxic oxygen species and degradative enzymes. The authors used an isolated, perfused rat liver preparation to determine whether PMNs might injure the liver. Livers from fasted rats were perfused with Krebs-Ringer bicarbonate buffer (pH 7.4) containing 3% bovine serum albumin (BSA) in a recirculating system. Rat peritoneal PMNs (4 x 10(8] or vehicle (Hank's balanced salt solution [HBSS], pH 7.35) were added, and liver injury was assessed 90 minutes later by release of alanine aminotransferase (ALT) into the perfusion medium and histopathologic analysis of liver sections. Perfusion of livers receiving only HBSS for 90 minutes resulted in a small increase in ALT activity in the perfusion medium but did not significantly alter histologic features of liver sections. Addition of unstimulated PMNs did not increase further the ALT activity and, with the exception of vascular neutrophilia, did not significantly change the histomorphology compared with controls. When PMNs activated with a combination of phorbol myristate acetate (PMA, 31 ng/ml) and lithocholate (100 mumol/l [micromolar]) were added to the perfusion system, however, livers released greater amounts of ALT than those perfused with PMA, lithocholate, and HBSS. Activated PMNs caused a transient reduction in flow of perfusion medium that lasted approximately 5 to 15 minutes. Liver sections had multifocal to coalescing foci of moderate to severe, acute hepatocellular necrosis associated with the areas of intense sinusoidal neutrophilia. In addition a second type of lesion was observed and was characterized by triangular foci of necrosis located adjacent to periportal regions of sinusoids or portal veins containing neutrophilic thrombi. These lesions were void of PMNs and were consistent with infarcts. A combination of superoxide dismutase and catalase added to the perfusion medium (500 U/ml each) prevented the elevation in ALT activity but not the transient reduction in flow. These results indicate that activated PMNs may cause liver injury by an oxygen radical-dependent mechanism. It is unclear whether PMN-derived oxygen radicals, hepatocellular-derived oxygen species resulting from reduced tissue perfusion and reperfusion, or both are involved in the pathogenesis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arthur M. J., Bentley I. S., Tanner A. R., Saunders P. K., Millward-Sadler G. H., Wright R. Oxygen-derived free radicals promote hepatic injury in the rat. Gastroenterology. 1985 Nov;89(5):1114–1122. doi: 10.1016/0016-5085(85)90218-5. [DOI] [PubMed] [Google Scholar]
- BEERS R. F., Jr, SIZER I. W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952 Mar;195(1):133–140. [PubMed] [Google Scholar]
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (first of two parts). N Engl J Med. 1978 Mar 23;298(12):659–668. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- Brigham K. L., Meyrick B. Interactions of granulocytes with the lungs. Circ Res. 1984 Jun;54(6):623–635. doi: 10.1161/01.res.54.6.623. [DOI] [PubMed] [Google Scholar]
- Dahm L. J., Hewett J. A., Roth R. A. Bile and bile salts potentiate superoxide anion release from activated, rat peritoneal neutrophils. Toxicol Appl Pharmacol. 1988 Aug;95(1):82–92. doi: 10.1016/s0041-008x(88)80010-3. [DOI] [PubMed] [Google Scholar]
- Dahm L. J., Roth R. A. Differential effects of lithocholate on rat neutrophil activation. J Leukoc Biol. 1990 Jun;47(6):551–560. doi: 10.1002/jlb.47.6.551. [DOI] [PubMed] [Google Scholar]
- Dahm L. J., Schultze A. E., Roth R. A. An antibody to neutrophils attenuates alpha-naphthylisothiocyanate-induced liver injury. J Pharmacol Exp Ther. 1991 Jan;256(1):412–420. [PubMed] [Google Scholar]
- Di Monte D., Bellomo G., Thor H., Nicotera P., Orrenius S. Menadione-induced cytotoxicity is associated with protein thiol oxidation and alteration in intracellular Ca2+ homeostasis. Arch Biochem Biophys. 1984 Dec;235(2):343–350. doi: 10.1016/0003-9861(84)90207-8. [DOI] [PubMed] [Google Scholar]
- Di Monte D., Ross D., Bellomo G., Eklöw L., Orrenius S. Alterations in intracellular thiol homeostasis during the metabolism of menadione by isolated rat hepatocytes. Arch Biochem Biophys. 1984 Dec;235(2):334–342. doi: 10.1016/0003-9861(84)90206-6. [DOI] [PubMed] [Google Scholar]
- Engerson T. D., McKelvey T. G., Rhyne D. B., Boggio E. B., Snyder S. J., Jones H. P. Conversion of xanthine dehydrogenase to oxidase in ischemic rat tissues. J Clin Invest. 1987 Jun;79(6):1564–1570. doi: 10.1172/JCI112990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferluga J., Allison A. C. Role of mononuclear infiltrating cells in pathogenesis of hepatitis. Lancet. 1978 Sep 16;2(8090):610–611. doi: 10.1016/s0140-6736(78)92828-3. [DOI] [PubMed] [Google Scholar]
- Holman J. M., Jr, Saba T. M. Hepatocyte injury during post-operative sepsis: activated neutrophils as potential mediators. J Leukoc Biol. 1988 Mar;43(3):193–203. doi: 10.1002/jlb.43.3.193. [DOI] [PubMed] [Google Scholar]
- Jaeschke H., Smith C. V., Mitchell J. R. Reactive oxygen species during ischemia-reflow injury in isolated perfused rat liver. J Clin Invest. 1988 Apr;81(4):1240–1246. doi: 10.1172/JCI113441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. J., Ward P. A. Biology of disease. Newer concepts in the pathogenesis of immune complex-induced tissue injury. Lab Invest. 1982 Sep;47(3):218–226. [PubMed] [Google Scholar]
- Krell H., Dietze E. Hemodynamic and metabolic responses to leukotriene C4 in isolated perfused rat liver. Hepatology. 1989 Sep;10(3):300–305. doi: 10.1002/hep.1840100308. [DOI] [PubMed] [Google Scholar]
- Kyle M. E., Nakae D., Sakaida I., Miccadei S., Farber J. L. Endocytosis of superoxide dismutase is required in order for the enzyme to protect hepatocytes from the cytotoxicity of hydrogen peroxide. J Biol Chem. 1988 Mar 15;263(8):3784–3789. [PubMed] [Google Scholar]
- Laskin D. L., Pilaro A. M., Ji S. Potential role of activated macrophages in acetaminophen hepatotoxicity. II. Mechanism of macrophage accumulation and activation. Toxicol Appl Pharmacol. 1986 Nov;86(2):216–226. doi: 10.1016/0041-008x(86)90052-9. [DOI] [PubMed] [Google Scholar]
- Laskin D. L., Pilaro A. M. Potential role of activated macrophages in acetaminophen hepatotoxicity. I. Isolation and characterization of activated macrophages from rat liver. Toxicol Appl Pharmacol. 1986 Nov;86(2):204–215. doi: 10.1016/0041-008x(86)90051-7. [DOI] [PubMed] [Google Scholar]
- Marotto M. E., Thurman R. G., Lemasters J. J. Early midzonal cell death during low-flow hypoxia in the isolated, perfused rat liver: protection by allopurinol. Hepatology. 1988 May-Jun;8(3):585–590. doi: 10.1002/hep.1840080325. [DOI] [PubMed] [Google Scholar]
- Mavier P., Preaux A. M., Guigui B., Lescs M. C., Zafrani E. S., Dhumeaux D. In vitro toxicity of polymorphonuclear neutrophils to rat hepatocytes: evidence for a proteinase-mediated mechanism. Hepatology. 1988 Mar-Apr;8(2):254–258. doi: 10.1002/hep.1840080211. [DOI] [PubMed] [Google Scholar]
- Orange R. P., Valentine M. D., Austen K. F. Release of slow-reacting substance of anaphylaxis in the rat: polymorphonuclear leukocyte. Science. 1967 Jul 21;157(3786):318–319. doi: 10.1126/science.157.3786.318. [DOI] [PubMed] [Google Scholar]
- Plaa G. L., Priestly B. G. Intrahepatic cholestasis induced by drugs and chemicals. Pharmacol Rev. 1976 Sep;28(3):207–273. [PubMed] [Google Scholar]
- REITMAN S., FRANKEL S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957 Jul;28(1):56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- Romson J. L., Hook B. G., Kunkel S. L., Abrams G. D., Schork M. A., Lucchesi B. R. Reduction of the extent of ischemic myocardial injury by neutrophil depletion in the dog. Circulation. 1983 May;67(5):1016–1023. doi: 10.1161/01.cir.67.5.1016. [DOI] [PubMed] [Google Scholar]
- Ruch R. J., Crist K. A., Klaunig J. E. Effects of culture duration on hydrogen peroxide-induced hepatocyte toxicity. Toxicol Appl Pharmacol. 1989 Sep 15;100(3):451–464. doi: 10.1016/0041-008x(89)90293-7. [DOI] [PubMed] [Google Scholar]
- Smith C. V. Evidence for participation of lipid peroxidation and iron in diquat-induced hepatic necrosis in vivo. Mol Pharmacol. 1987 Sep;32(3):417–422. [PubMed] [Google Scholar]
- Till G. O., Beauchamp C., Menapace D., Tourtellotte W., Jr, Kunkel R., Johnson K. J., Ward P. A. Oxygen radical dependent lung damage following thermal injury of rat skin. J Trauma. 1983 Apr;23(4):269–277. doi: 10.1097/00005373-198304000-00001. [DOI] [PubMed] [Google Scholar]
- Younes M., Strubelt O. The involvement of reactive oxygen species in hypoxic injury to rat liver. Res Commun Chem Pathol Pharmacol. 1988 Mar;59(3):369–381. [PubMed] [Google Scholar]
- Zhong Z., Lemasters J. J., Thurman R. G. Role of purines and xanthine oxidase in reperfusion injury in perfused rat liver. J Pharmacol Exp Ther. 1989 Aug;250(2):470–475. [PubMed] [Google Scholar]









