Abstract
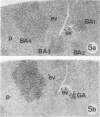
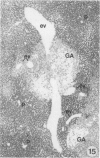
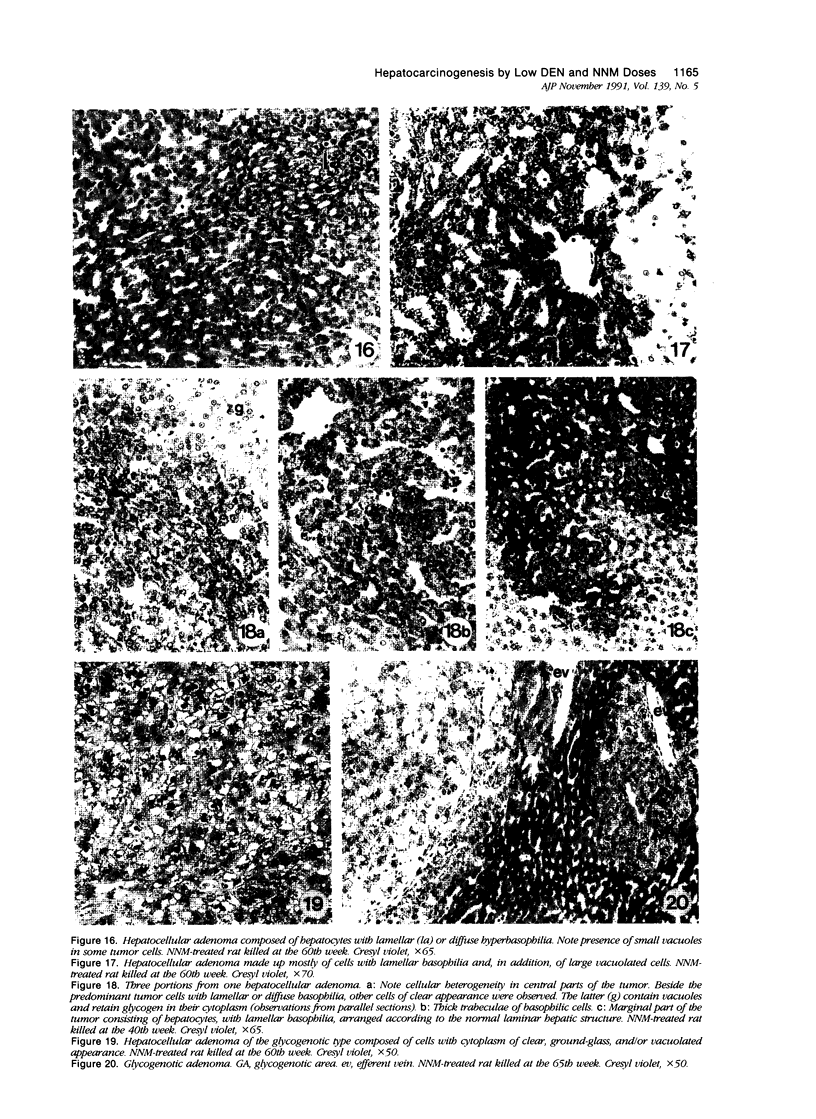
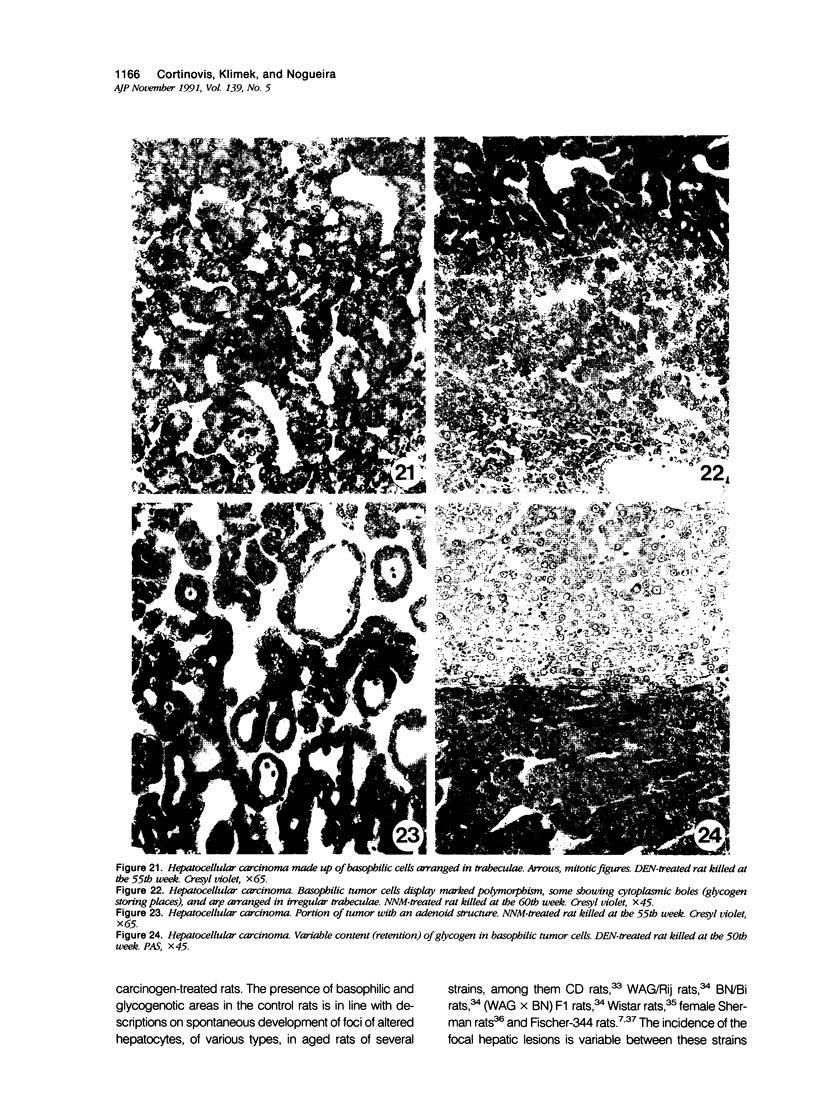
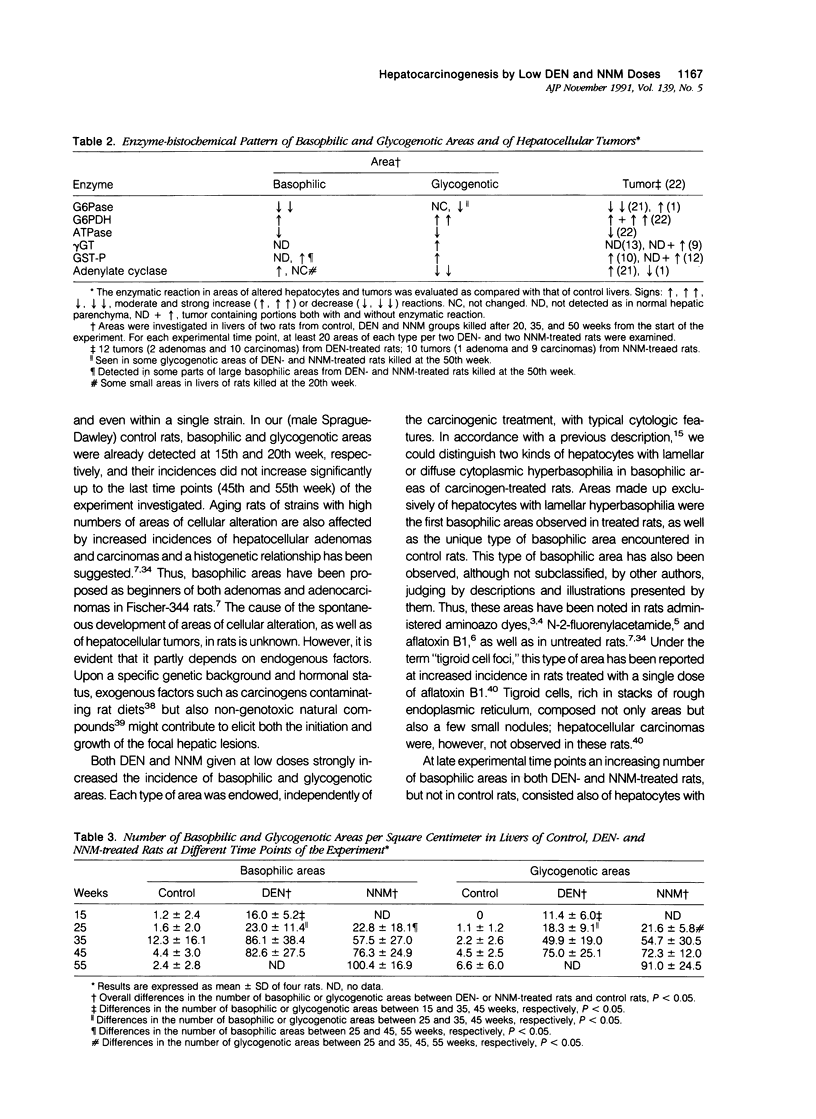
The development of hepatocellular tumors was investigated with histological, histochemical, and morphometrical methods in male Sprague-Dawley rats continuously administered N-nitrosodiethylamine (DEN) or N-nitrosomorpholine (NNM) in the drinking water at low doses (0.5 mg DEN/100 ml; 1 mg NNM/100 ml). Groups of control, DEN-, and NNM-treated rats were investigated at 5-week intervals. Similar results were obtained in DEN- and NNM-treated rats. Two types of areas composed of basophilic or glycogenotic hepatocytes were observed preceding the appearance of hepatocellular adenomas and carcinomas. Besides their cytologic differences, the basophilic and glycogenotic areas induced displayed distinct histochemical features. Both types of areas were detected simultaneously and increased in parallel with time to a similar incidence, but basophilic areas reached larger sizes than the glycogenotic ones. Furthermore, each type of area, which clustered around and along efferent veins, was differently linked to tumorigenesis. Basophilic areas frequently developed into basophilic adenomas and trabecular carcinomas through a characteristic sequence. Early basophilic areas consisted of hepatocytes with lamellar cytoplasmic hyperbasophilia and exhibited the normal laminar liver structure. With time, an increasing number of basophilic areas also contained hepatocytes with powdered diffuse hyperbasophilia, which frequently were arranged in thick trabeculae, showed abundant mitotic figures, and invaded efferent veins. Neither such signs of malignancy nor conversion into basophilic areas or tumors could be established for areas of clear and acidophilic glycogenotic hepatocytes. However, a few small glycogenotic adenomas probably developed from glycogenotic areas. Our data thus underline the central role of basophilic areas for hepatocarcinogenesis. Moreover, taking into account the data from other experiments, it seems likely that although glycogenotic areas may be associated with the application of some carcinogens at high doses, they are not obligatory precursors of hepatocellular tumors.
Full text
PDF



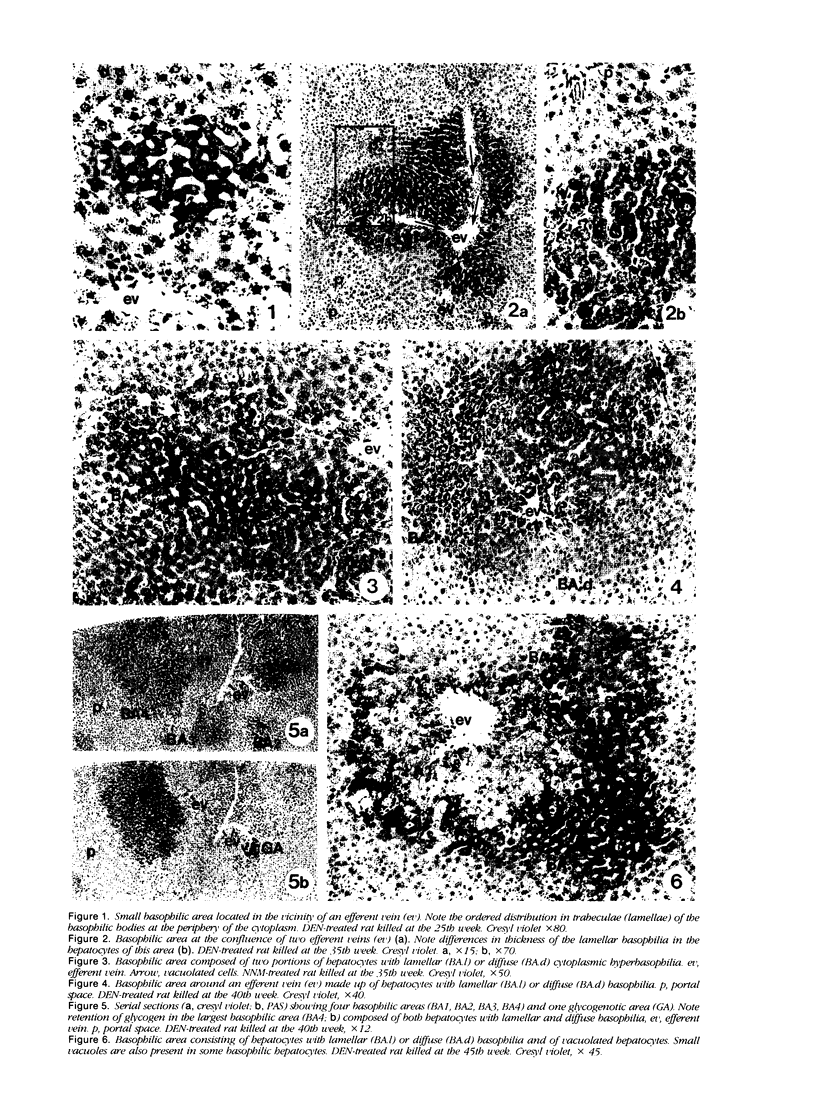
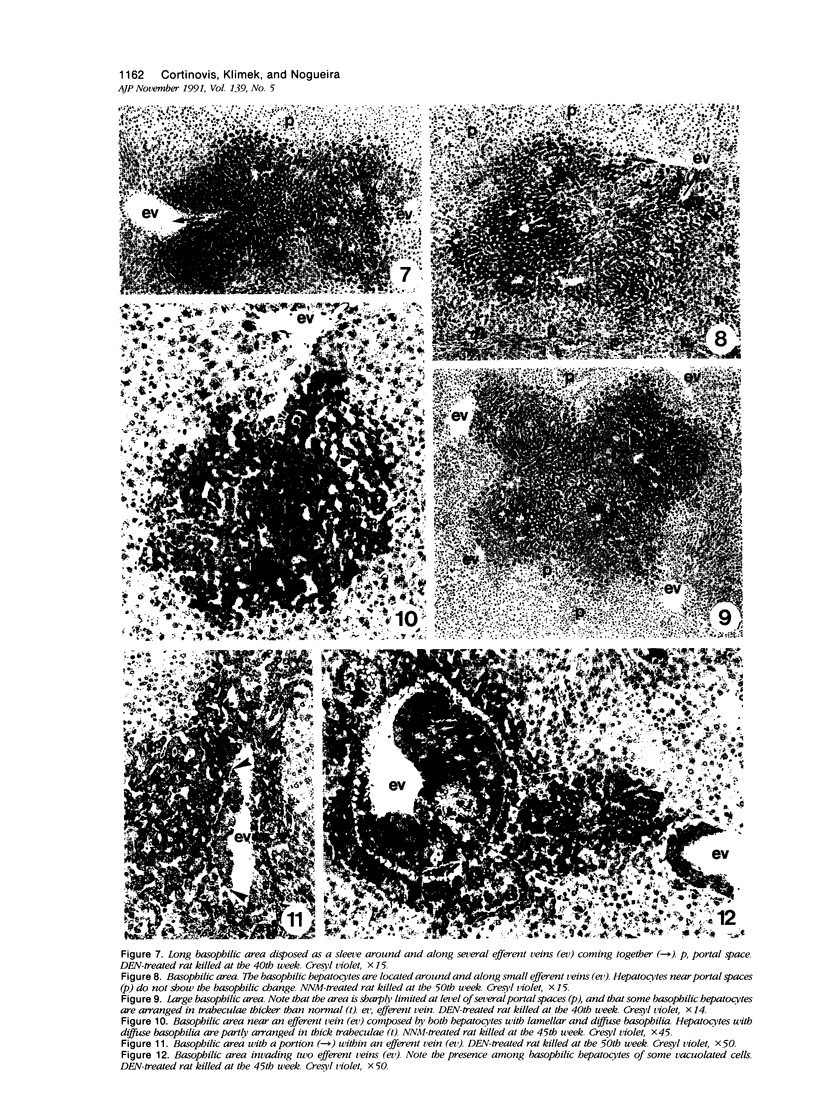
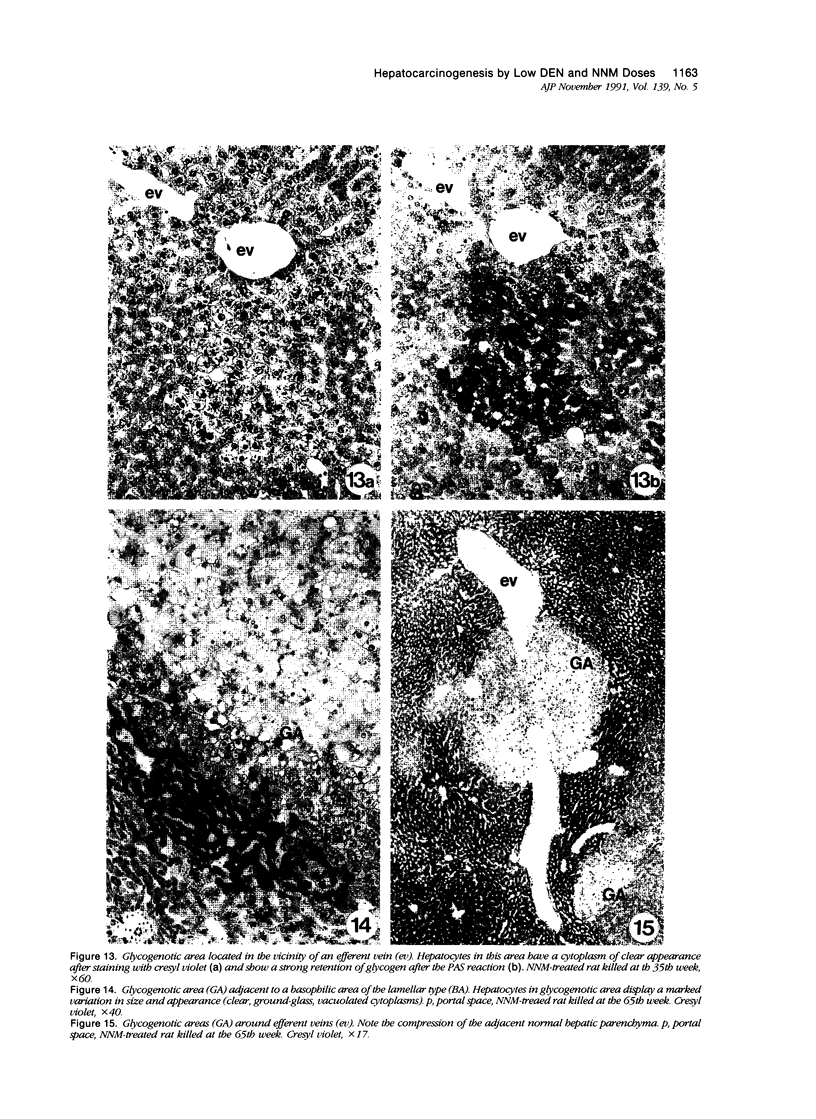





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N., Gold L. S. Too many rodent carcinogens: mitogenesis increases mutagenesis. Science. 1990 Aug 31;249(4972):970–971. doi: 10.1126/science.2136249. [DOI] [PubMed] [Google Scholar]
- Audisio R. A., Bombelli L., Lombardi L., Andreola S. A clinico-pathologic study of clear-cell hepatocellular carcinoma. Tumori. 1987 Aug 31;73(4):389–395. doi: 10.1177/030089168707300412. [DOI] [PubMed] [Google Scholar]
- Babcock M. B., Cardell R. R., Jr Hepatic glycogen patterns in fasted and fed rats. Am J Anat. 1974 Jul;140(3):299–337. doi: 10.1002/aja.1001400302. [DOI] [PubMed] [Google Scholar]
- Bannasch P., Benner U., Enzmann H., Hacker H. J. Tigroid cell foci and neoplastic nodules in the liver of rats treated with a single dose of aflatoxin B1. Carcinogenesis. 1985 Nov;6(11):1641–1648. doi: 10.1093/carcin/6.11.1641. [DOI] [PubMed] [Google Scholar]
- Butler W. H., Hempsall V., Stewart M. G. Histochemical studies on the early proliferative lesion induced in the rat liver by aflatoxin. J Pathol. 1981 Apr;133(4):325–340. doi: 10.1002/path.1711330405. [DOI] [PubMed] [Google Scholar]
- Daoust R., Calamai R. Hyperbasophilic foci as sites of neoplastic transformation in hepatic parenchyma. Cancer Res. 1971 Sep;31(9):1290–1296. [PubMed] [Google Scholar]
- Edwards G. S., Fox J. G., Policastro P., Goff U., Wolf M. H., Fine D. H. Volatile nitrosamine contamination of laboratory animal diets. Cancer Res. 1979 May;39(5):1857–1858. [PubMed] [Google Scholar]
- Ehemann V., Mayer D., Hacker H. J., Bannasch P. Loss of adenylate cyclase activity in preneoplastic and neoplastic lesions induced in rat liver by N-nitrosomorpholine. Carcinogenesis. 1986 Apr;7(4):567–573. doi: 10.1093/carcin/7.4.567. [DOI] [PubMed] [Google Scholar]
- Goldfarb S., Pugh T. D. Enzyme histochemical phenotypes in primary hepatocellular carcinomas. Cancer Res. 1981 Jun;41(6):2092–2095. [PubMed] [Google Scholar]
- Goldfarb S., Pugh T. D., Koen H., He Y. Z. Preneoplastic and neoplastic progression during hepatocarcinogenesis in mice injected with diethylnitrosamine in infancy. Environ Health Perspect. 1983 Apr;50:149–161. doi: 10.1289/ehp.8350149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker H. J., Moore M. A., Mayer D., Bannasch P. Correlative histochemistry of some enzymes of carbohydrate metabolism in preneoplastic and neoplastic lesions in the rat liver. Carcinogenesis. 1982;3(11):1265–1272. doi: 10.1093/carcin/3.11.1265. [DOI] [PubMed] [Google Scholar]
- Kalengayi M. M., Ronchi G., Desmet V. J. Histochemistry of gamma-glutamyl transpeptidase in rat liver during aflatoxin B1-induced carcinogenesis. J Natl Cancer Inst. 1975 Sep;55(3):579–588. doi: 10.1093/jnci/55.3.579. [DOI] [PubMed] [Google Scholar]
- Karasaki S. The fine structure of proliferating cells in preneoplastic rat livers during azo-dye carcinogenesis. J Cell Biol. 1969 Feb;40(2):322–335. doi: 10.1083/jcb.40.2.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimbrough R. D., Squire R. A., Linder R. E., Strandberg J. D., Montalli R. J., Burse V. W. Induction of liver tumor in Sherman strain female rats by polychlorinated biphenyl aroclor 1260. J Natl Cancer Inst. 1975 Dec;55(6):1453–1459. doi: 10.1093/jnci/55.6.1453. [DOI] [PubMed] [Google Scholar]
- Lamers W. H., Hilberts A., Furt E., Smith J., Jonges G. N., van Noorden C. J., Janzen J. W., Charles R., Moorman A. F. Hepatic enzymic zonation: a reevaluation of the concept of the liver acinus. Hepatology. 1989 Jul;10(1):72–76. doi: 10.1002/hep.1840100115. [DOI] [PubMed] [Google Scholar]
- Maguire S., Rabes H. M. Intralobular distribution of preneoplastic foci in rat liver after a single dose of N-methyl-N-nitrosourea (MNU) following partial hepatectomy. Carcinogenesis. 1989 May;10(5):871–874. doi: 10.1093/carcin/10.5.871. [DOI] [PubMed] [Google Scholar]
- Matsumoto T., Kawakami M. The unit-concept of hepatic parenchyma--a re-examination based on angioarchitectural studies. Acta Pathol Jpn. 1982;32 (Suppl 2):285–314. [PubMed] [Google Scholar]
- Mayer D., Ehemann V., Hacker H. J., Klimek F., Bannasch P. Specificity of cytochemical demonstration of adenylate cyclase in liver using adenylate-(beta, gamma-methylene) diphosphate as substrate. Histochemistry. 1985;82(2):135–140. doi: 10.1007/BF00708197. [DOI] [PubMed] [Google Scholar]
- Meijer A. E., de Vries G. P. Semipermeable membranes for improving the histochemical demonstration of enzyme activities in tissue sections. IV. Glucose 6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase (decarboxylating). Histochemistry. 1974;40(4):349–359. doi: 10.1007/BF00495042. [DOI] [PubMed] [Google Scholar]
- Moore M. A., Kitagawa T. Hepatocarcinogenesis in the rat: the effect of promoters and carcinogens in vivo and in vitro. Int Rev Cytol. 1986;101:125–173. doi: 10.1016/s0074-7696(08)60248-x. [DOI] [PubMed] [Google Scholar]
- Moore M. A., Mayer D., Bannasch P. The dose dependence and sequential appearance of putative preneoplastic populations induced in the rat liver by stop experiments with N-nitrosomorpholine. Carcinogenesis. 1982;3(12):1429–1436. doi: 10.1093/carcin/3.12.1429. [DOI] [PubMed] [Google Scholar]
- Ogawa K., Onoé T., Takeuchi M. Spontaneous occurrence of gamma-glutamyl transpeptidase-positive hepatocytic foci in 105-week-old Wistar and 72-week-old Fischer 34 male rats. J Natl Cancer Inst. 1981 Aug;67(2):407–412. [PubMed] [Google Scholar]
- Popp J. A., Scortichini B. H., Garvey L. K. Quantitative evaluation of hepatic foci of cellular alteration occurring spontaneously in Fischer-344 rats. Fundam Appl Toxicol. 1985 Apr;5(2):314–319. doi: 10.1016/0272-0590(85)90079-x. [DOI] [PubMed] [Google Scholar]
- Reuber M. D. Development of preneoplastic and neoplastic lesions of the liver in male rats given 0.025 percent N-2-fluorenyldiacetamide. J Natl Cancer Inst. 1965 Jun;34(6):697–723. [PubMed] [Google Scholar]
- Satoh K., Kitahara A., Soma Y., Inaba Y., Hatayama I., Sato K. Purification, induction, and distribution of placental glutathione transferase: a new marker enzyme for preneoplastic cells in the rat chemical hepatocarcinogenesis. Proc Natl Acad Sci U S A. 1985 Jun;82(12):3964–3968. doi: 10.1073/pnas.82.12.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer A., Kunze E. Enzymhistochemische und autoradiographische Untersuchungen während der Cancerisierung der Rattenleber mit Diäthylnitrosamin. Z Krebsforsch. 1968;70(3):252–266. [PubMed] [Google Scholar]
- Schmitz-Moormann P., Gedigk P., Dharamadhach A. Histologische und histochemische Frühveränderungen bei der experimentellen Erzeugung von Lebercarcinomen durch Diäthylnitrosamin. Z Krebsforsch Klin Onkol Cancer Res Clin Oncol. 1972;77(1):9–16. doi: 10.1007/BF00284349. [DOI] [PubMed] [Google Scholar]
- Teutsch H. F. Improved method for the histochemical demonstration of glucose-6-phosphatase activity. Histochemistry. 1978 Aug 25;57(2):107–117. doi: 10.1007/BF00496675. [DOI] [PubMed] [Google Scholar]
- Ulland B. M., Page N. P., Squire R. A., Weisburger E. K., Cypher R. L. A carcinogenicity assay of Mirex in Charles River CD rats. J Natl Cancer Inst. 1977 Jan;58(1):133–140. doi: 10.1093/jnci/58.1.133. [DOI] [PubMed] [Google Scholar]
- Vesselinovitch S. D., Hacker H. J., Bannasch P. Histochemical characterization of focal hepatic lesions induced by single diethylnitrosamine treatment in infant mice. Cancer Res. 1985 Jun;45(6):2774–2780. [PubMed] [Google Scholar]
- Vesselinovitch S. D., Mihailovich N. Kinetics of diethylnitrosamine hepatocarcinogenesis in the infant mouse. Cancer Res. 1983 Sep;43(9):4253–4259. [PubMed] [Google Scholar]
- Ward J. M. Morphology of foci of altered hepatocytes and naturally-occurring hepatocellular tumors in F344 rats. Virchows Arch A Pathol Anat Histol. 1981;390(3):339–345. doi: 10.1007/BF00496563. [DOI] [PubMed] [Google Scholar]
- Williams G. M., Klaiber M., Parker S. E., Farber E. Nature of early appearing, carcinogen-induced liver lesions to iron accumulation. J Natl Cancer Inst. 1976 Jul;57(1):157–165. doi: 10.1093/jnci/57.1.157. [DOI] [PubMed] [Google Scholar]
- Wu P. C., Lai C. L., Lam K. C., Lok A. S., Lin H. J. Clear cell carcinoma of liver. An ultrastructural study. Cancer. 1983 Aug 1;52(3):504–507. doi: 10.1002/1097-0142(19830801)52:3<504::aid-cncr2820520321>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]