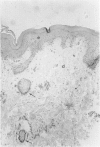
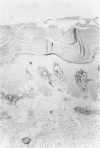
Abstract
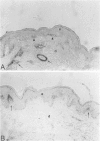
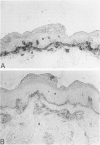
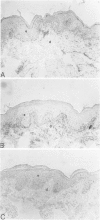
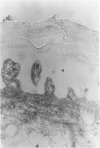
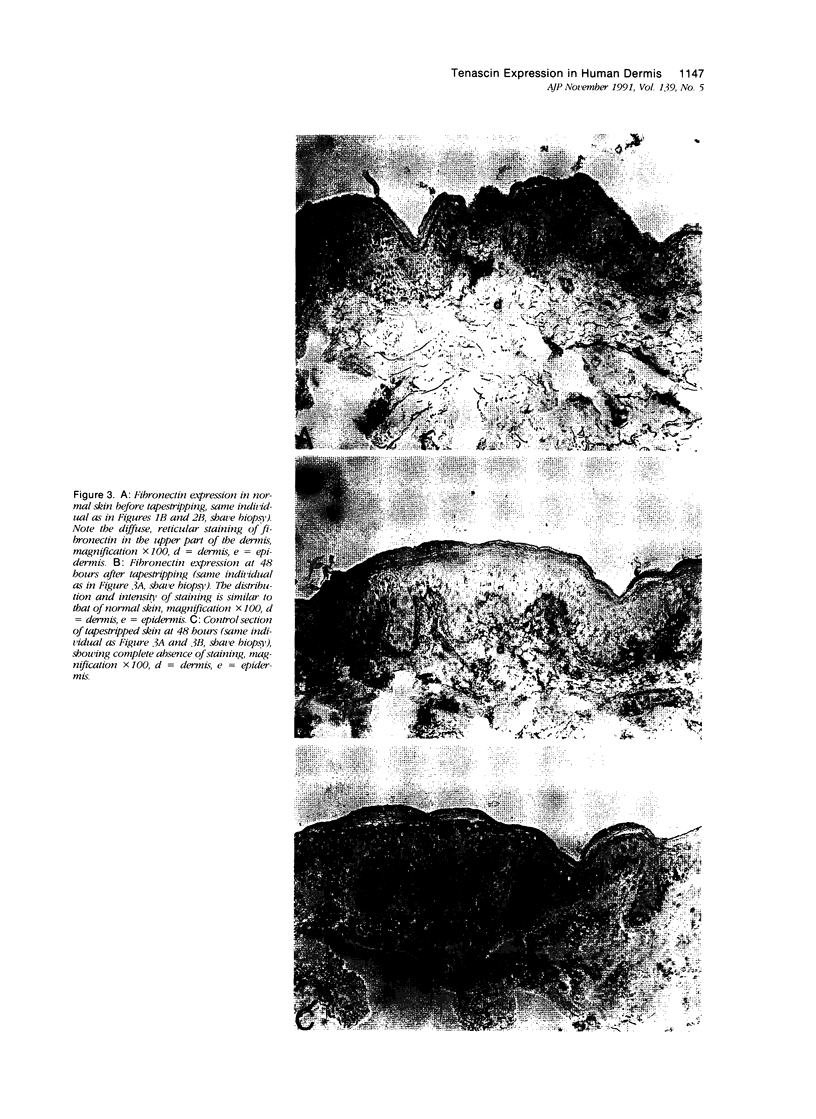
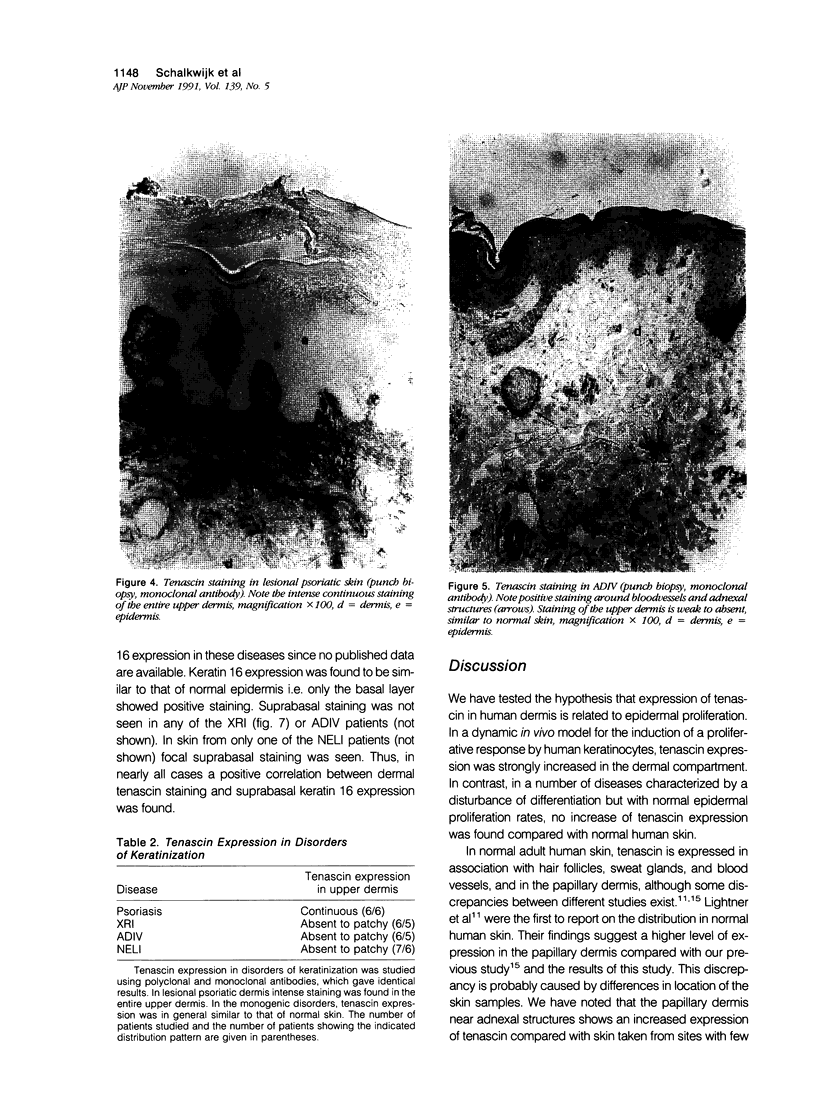
The extracellular matrix glycoprotein tenascin is sparsely distributed in normal human dermis. The authors have shown that in a number of skin diseases (psoriasis, skin tumors), tenascin expression is strongly increased. In this immunohistochemical study, using polyclonal and monoclonal antisera, we have tested the hypothesis that tenascin expression in vivo is linked to epidermal proliferation. Using the sellotape stripping model in normal human skin, which causes a rapid recruitment of keratinocytes into the cell cycle, induction of tenascin expression was found in the upper dermis within 24 hours after stripping. In contrast, in normoproliferative monogenic disorders of keratinization (X-linked recessive ichthyosis, autosomal dominant ichthyosis vulgaris, non-erythrodermic lamellar ichthyosis), no increase in tenascin expression was found compared with normal skin. These findings demonstrate a relationship between epidermal proliferation and metabolic alterations in the dermal compartment.
Full text
PDF

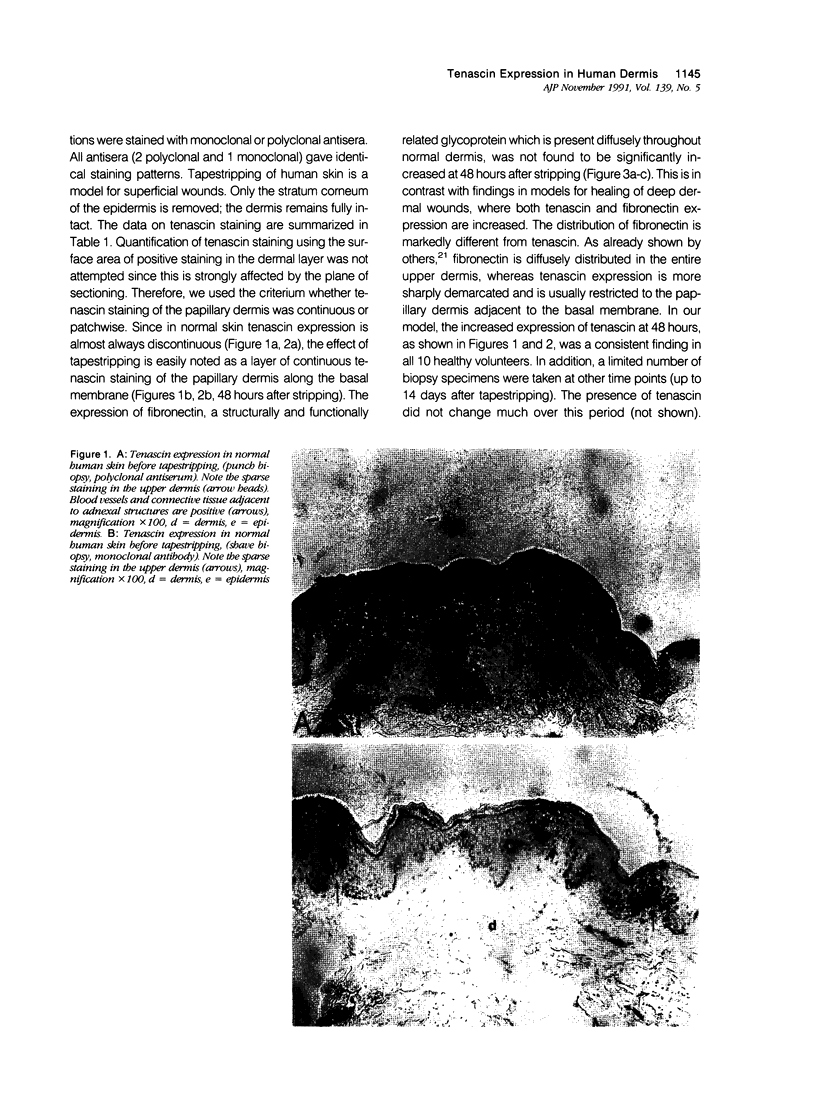
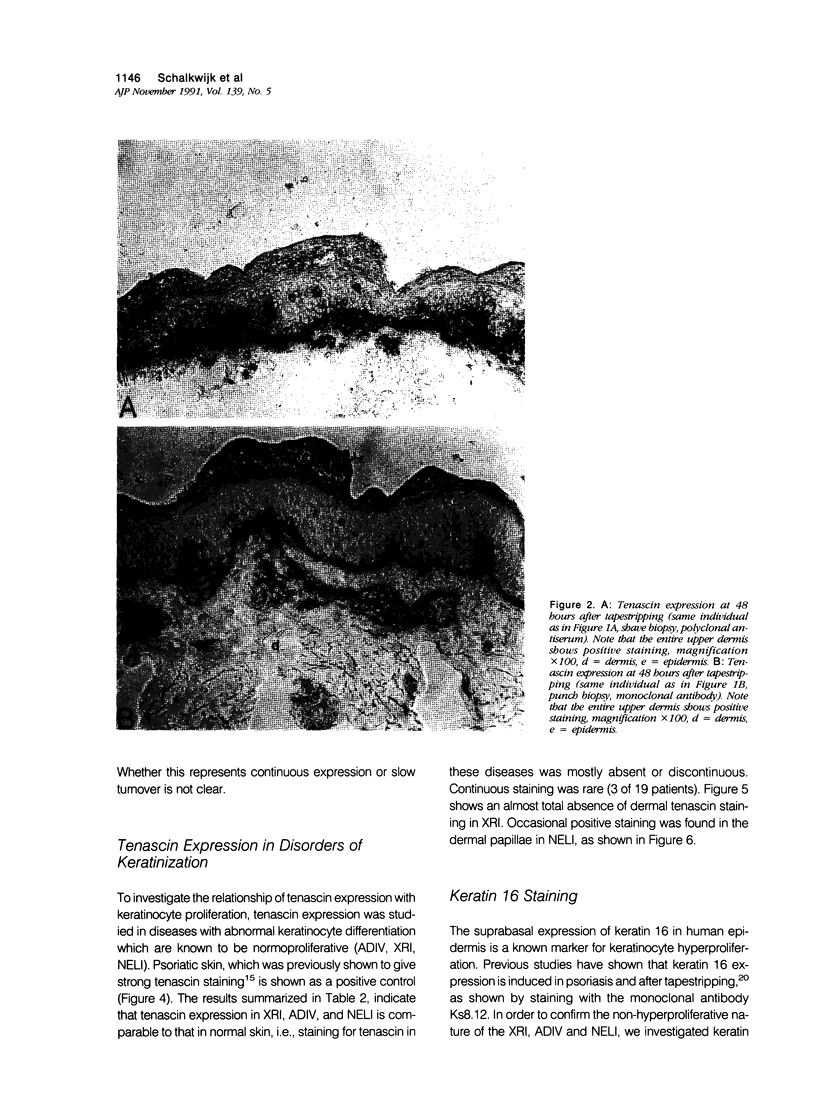


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anbazhagan R., Sakakura T., Gusterson B. A. The distribution of immuno-reactive tenascin in the epithelial-mesenchymal junctional areas of benign and malignant squamous epithelia. Virchows Arch B Cell Pathol Incl Mol Pathol. 1990;59(1):59–63. doi: 10.1007/BF02899388. [DOI] [PubMed] [Google Scholar]
- Boezeman J. B., Bauer F. W., de Grood R. M. Flow cytometric analysis of the recruitment of G0 cells in human epidermis in vivo following tape stripping. Cell Tissue Kinet. 1987 Jan;20(1):99–107. doi: 10.1111/j.1365-2184.1987.tb01086.x. [DOI] [PubMed] [Google Scholar]
- Chiquet-Ehrismann R., Kalla P., Pearson C. A., Beck K., Chiquet M. Tenascin interferes with fibronectin action. Cell. 1988 May 6;53(3):383–390. doi: 10.1016/0092-8674(88)90158-4. [DOI] [PubMed] [Google Scholar]
- Chiquet-Ehrismann R., Mackie E. J., Pearson C. A., Sakakura T. Tenascin: an extracellular matrix protein involved in tissue interactions during fetal development and oncogenesis. Cell. 1986 Oct 10;47(1):131–139. doi: 10.1016/0092-8674(86)90374-0. [DOI] [PubMed] [Google Scholar]
- Chiquet M., Fambrough D. M. Chick myotendinous antigen. II. A novel extracellular glycoprotein complex consisting of large disulfide-linked subunits. J Cell Biol. 1984 Jun;98(6):1937–1946. doi: 10.1083/jcb.98.6.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. A. Fibronectin in the skin. J Invest Dermatol. 1983 Dec;81(6):475–479. doi: 10.1111/1523-1747.ep12522718. [DOI] [PubMed] [Google Scholar]
- Erickson H. P., Inglesias J. L. A six-armed oligomer isolated from cell surface fibronectin preparations. Nature. 1984 Sep 20;311(5983):267–269. doi: 10.1038/311267a0. [DOI] [PubMed] [Google Scholar]
- Gulcher J. R., Nies D. E., Marton L. S., Stefansson K. An alternatively spliced region of the human hexabrachion contains a repeat of potential N-glycosylation sites. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1588–1592. doi: 10.1073/pnas.86.5.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman S., Crossin K. L., Edelman G. M. Molecular forms, binding functions, and developmental expression patterns of cytotactin and cytotactin-binding proteoglycan, an interactive pair of extracellular matrix molecules. J Cell Biol. 1988 Feb;106(2):519–532. doi: 10.1083/jcb.106.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones F. S., Burgoon M. P., Hoffman S., Crossin K. L., Cunningham B. A., Edelman G. M. A cDNA clone for cytotactin contains sequences similar to epidermal growth factor-like repeats and segments of fibronectin and fibrinogen. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2186–2190. doi: 10.1073/pnas.85.7.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse J., Keilhauer G., Faissner A., Timpl R., Schachner M. The J1 glycoprotein--a novel nervous system cell adhesion molecule of the L2/HNK-1 family. Nature. 1985 Jul 11;316(6024):146–148. doi: 10.1038/316146a0. [DOI] [PubMed] [Google Scholar]
- Lightner V. A., Gumkowski F., Bigner D. D., Erickson H. P. Tenascin/hexabrachion in human skin: biochemical identification and localization by light and electron microscopy. J Cell Biol. 1989 Jun;108(6):2483–2493. doi: 10.1083/jcb.108.6.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie E. J., Chiquet-Ehrismann R., Pearson C. A., Inaguma Y., Taya K., Kawarada Y., Sakakura T. Tenascin is a stromal marker for epithelial malignancy in the mammary gland. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4621–4625. doi: 10.1073/pnas.84.13.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie E. J., Halfter W., Liverani D. Induction of tenascin in healing wounds. J Cell Biol. 1988 Dec;107(6 Pt 2):2757–2767. doi: 10.1083/jcb.107.6.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie E. J., Thesleff I., Chiquet-Ehrismann R. Tenascin is associated with chondrogenic and osteogenic differentiation in vivo and promotes chondrogenesis in vitro. J Cell Biol. 1987 Dec;105(6 Pt 1):2569–2579. doi: 10.1083/jcb.105.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McComb R. D., Moul J. M., Bigner D. D. Distribution of type VI collagen in human gliomas: comparison with fibronectin and glioma-mesenchymal matrix glycoprotein. J Neuropathol Exp Neurol. 1987 Nov;46(6):623–633. doi: 10.1097/00005072-198711000-00002. [DOI] [PubMed] [Google Scholar]
- Schalkwijk J., Van Vlijmen I., Oosterling B., Perret C., Koopman R., Van den Born J., Mackie E. J. Tenascin expression in hyperproliferative skin diseases. Br J Dermatol. 1991 Jan;124(1):13–20. doi: 10.1111/j.1365-2133.1991.tb03276.x. [DOI] [PubMed] [Google Scholar]
- Stamp G. W. Tenascin distribution in basal cell carcinomas. J Pathol. 1989 Nov;159(3):225–229. doi: 10.1002/path.1711590309. [DOI] [PubMed] [Google Scholar]
- Thesleff I., Mackie E., Vainio S., Chiquet-Ehrismann R. Changes in the distribution of tenascin during tooth development. Development. 1987 Oct;101(2):289–296. doi: 10.1242/dev.101.2.289. [DOI] [PubMed] [Google Scholar]
- de Mare S., Van Erp P. E., van de Kerkhof P. C. Epidermal hyperproliferation assessed by the monoclonal antibody Ks8.12 on frozen sections. J Invest Dermatol. 1989 Jan;92(1):130–131. doi: 10.1111/1523-1747.ep13071345. [DOI] [PubMed] [Google Scholar]