Abstract
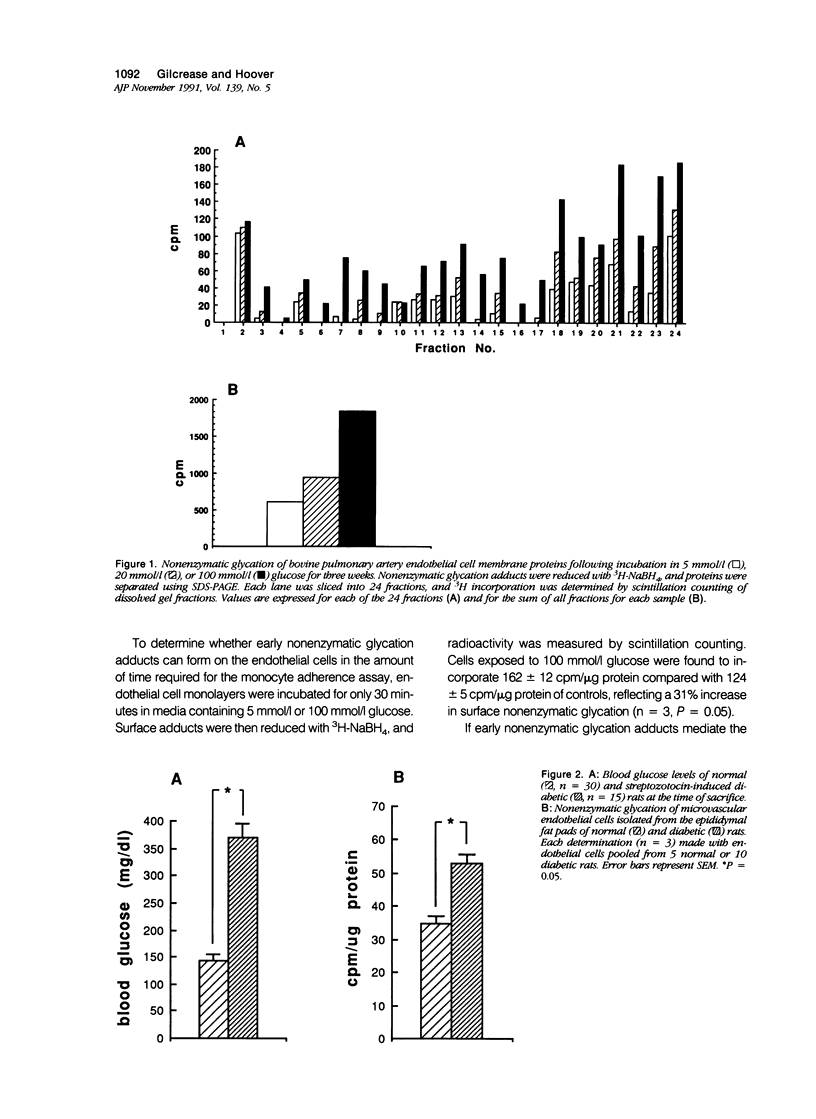
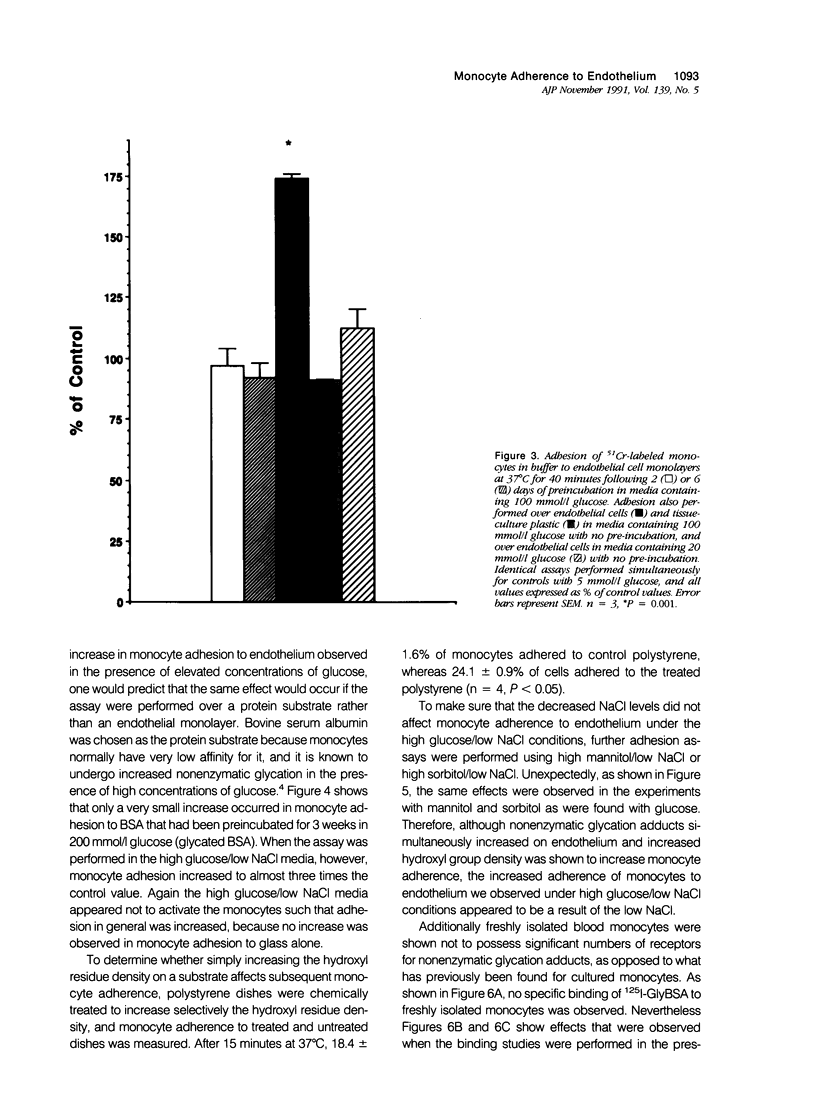
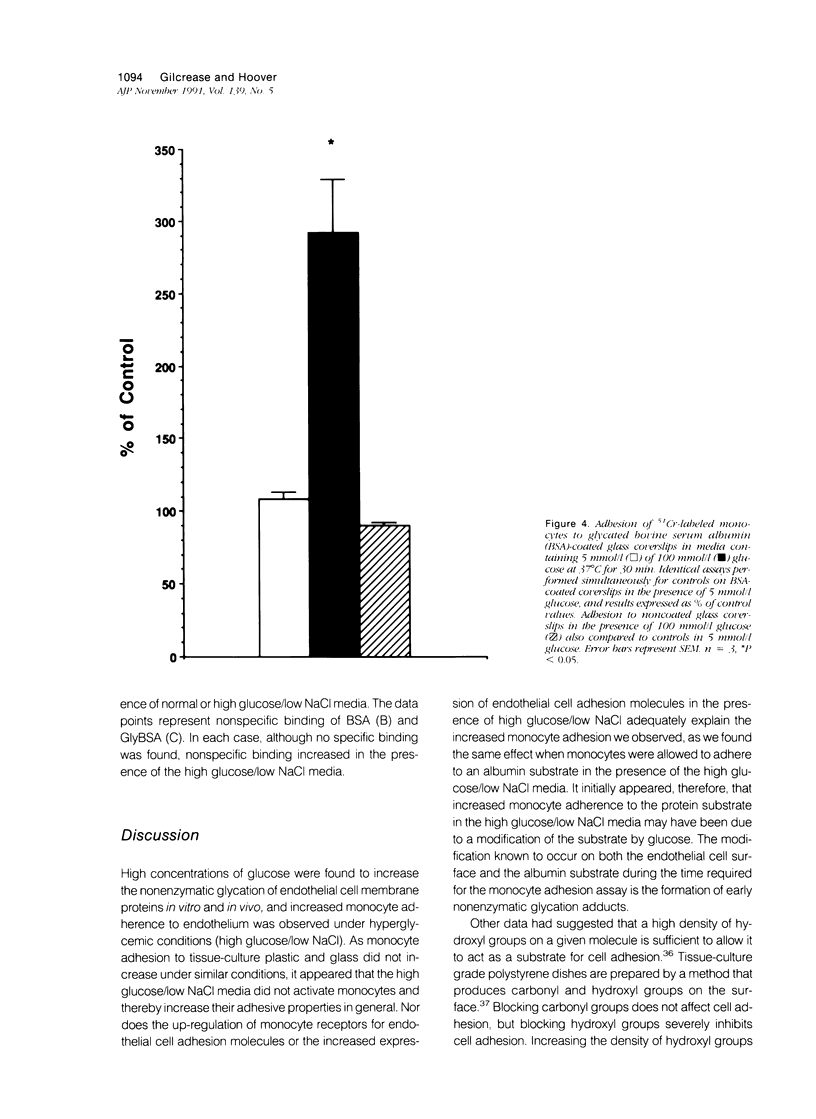
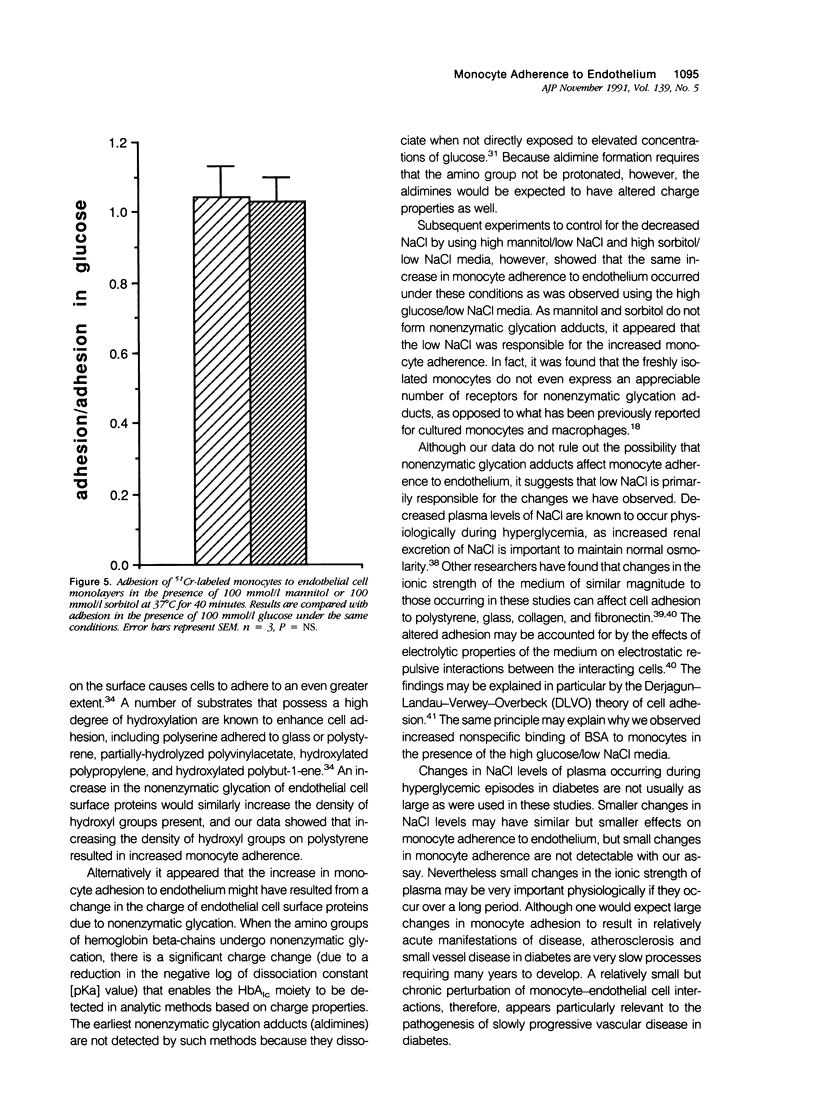
Increased nonenzymatic glycation of proteins has been implicated in the pathogenesis of diabetic vascular disease. The authors have shown by 3H-NaBH4 reduction of nonenzymatic glycation adducts that endothelial cell membrane proteins undergo increased nonenzymatic glycation in vitro when exposed to elevated concentrations of glucose. Increased nonenzymatic glycation also was found in vivo for microvascular endothelial cells isolated from streptozotocin-induced diabetic rats compared with control rats. Cultured monocytes have previously been reported to express receptors for certain nonenzymatic glycation adducts. The authors have further investigated whether monocyte interactions with endothelium are altered by the presence of nonenzymatic glycation adducts on endothelium. Adherence assays were performed in the presence of elevated concentrations of glucose with decreased NaCl levels to maintain normal osmolarity (as occurs physiologically). Although monocyte adherence to endothelium and levels of early nonenzymatic glycation adducts increased under these conditions, the increased adherence appears to be due to the altered NaCl levels. In fact, freshly isolated monocytes (in contrast to what has been found for macrophages and activated monocytes) were shown not to express appreciable numbers of receptors for nonenzymatic glycation adducts.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arieff A. I., Carroll H. J. Nonketotic hyperosmolar coma with hyperglycemia: clinical features, pathophysiology, renal function, acid-base balance, plasma-cerebrospinal fluid equilibria and the effects of therapy in 37 cases. Medicine (Baltimore) 1972 Mar;51(2):73–94. doi: 10.1097/00005792-197203000-00001. [DOI] [PubMed] [Google Scholar]
- Bookchin R. M., Gallop P. M. Structure of hemoglobin AIc: nature of the N-terminal beta chain blocking group. Biochem Biophys Res Commun. 1968 Jul 11;32(1):86–93. doi: 10.1016/0006-291x(68)90430-0. [DOI] [PubMed] [Google Scholar]
- Brownlee M., Vlassara H., Cerami A. Inhibition of heparin-catalyzed human antithrombin III activity by nonenzymatic glycosylation. Possible role in fibrin deposition in diabetes. Diabetes. 1984 Jun;33(6):532–535. doi: 10.2337/diab.33.6.532. [DOI] [PubMed] [Google Scholar]
- Brownlee M., Vlassara H., Cerami A. Nonenzymatic glycosylation reduces the susceptibility of fibrin to degradation by plasmin. Diabetes. 1983 Jul;32(7):680–684. doi: 10.2337/diab.32.7.680. [DOI] [PubMed] [Google Scholar]
- Cerami A., Vlassara H., Brownlee M. Protein glycosylation and the pathogenesis of atherosclerosis. Metabolism. 1985 Dec;34(12 Suppl 1):37–42. doi: 10.1016/s0026-0495(85)80008-1. [DOI] [PubMed] [Google Scholar]
- Cohen M. P., Urdanivia E., Surma M., Ciborowski C. J. Nonenzymatic glycosylation of basement membranes: in vitro studies. Diabetes. 1981 May;30(5):367–371. doi: 10.2337/diab.30.5.367. [DOI] [PubMed] [Google Scholar]
- Curtis A. S., Forrester J. V. Cell interactions: some critical reappraisals. Biochem Soc Trans. 1984 Jun;12(3):538–539. doi: 10.1042/bst0120538. [DOI] [PubMed] [Google Scholar]
- Curtis A. S., Forrester J. V., McInnes C., Lawrie F. Adhesion of cells to polystyrene surfaces. J Cell Biol. 1983 Nov;97(5 Pt 1):1500–1506. doi: 10.1083/jcb.97.5.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day J. F., Thorpe S. R., Baynes J. W. Nonenzymatically glucosylated albumin. In vitro preparation and isolation from normal human serum. J Biol Chem. 1979 Feb 10;254(3):595–597. [PubMed] [Google Scholar]
- Eble A. S., Thorpe S. R., Baynes J. W. Nonenzymatic glucosylation and glucose-dependent cross-linking of protein. J Biol Chem. 1983 Aug 10;258(15):9406–9412. [PubMed] [Google Scholar]
- Facchini P. J., Neumann A. W., DiCosmo F. Adhesion of suspension-cultured Catharanthus roseus cells to surfaces: effect of pH, ionic strength, and cation valency. Biomaterials. 1989 Jul;10(5):318–324. doi: 10.1016/0142-9612(89)90072-0. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Johnson A. R. Human pulmonary endothelial cells in culture. Activities of cells from arteries and cells from veins. J Clin Invest. 1980 Apr;65(4):841–850. doi: 10.1172/JCI109736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketis N. V., Hoover R. L., Karnovsky M. J. Isolation of bovine aortic endothelial cell plasma membranes: identification of membrane-associated cytoskeletal proteins. J Cell Physiol. 1986 Aug;128(2):162–170. doi: 10.1002/jcp.1041280205. [DOI] [PubMed] [Google Scholar]
- Koenig R. J., Cerami A. Hemoglobin A Ic and diabetes mellitus. Annu Rev Med. 1980;31:29–34. doi: 10.1146/annurev.me.31.020180.000333. [DOI] [PubMed] [Google Scholar]
- Komazaki S. Effects of salts in promoting the adhesion of amphibian gastrula cells. J Exp Zool. 1989 Apr;250(1):40–48. doi: 10.1002/jez.1402500106. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Magargal W. W., Dickinson E. S., Slakey L. L. Distribution of membrane marker enzymes in cultured arterial endothelial and smooth muscle cells. The subcellular location of oleoyl-CoA:1-acyl-sn-glycero-3-phosphocholine acyltransferase. J Biol Chem. 1978 Nov 25;253(22):8311–8318. [PubMed] [Google Scholar]
- McVerry B. A., Thorpe S., Joe F., Gaffney P., Huehns E. R. Non-enzymatic glucosylation of fibrinogen. Haemostasis. 1981;10(5):261–270. doi: 10.1159/000214409. [DOI] [PubMed] [Google Scholar]
- Miller J. A., Gravallese E., Bunn H. F. Nonenzymatic glycosylation of erythrocyte membrane proteins. Relevance to diabetes. J Clin Invest. 1980 Apr;65(4):896–901. doi: 10.1172/JCI109743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnier V. M., Stevens V. J., Cerami A. Nonenzymatic glycosylation, sulfhydryl oxidation, and aggregation of lens proteins in experimental sugar cataracts. J Exp Med. 1979 Nov 1;150(5):1098–1107. doi: 10.1084/jem.150.5.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perutz M. F. Regulation of oxygen affinity of hemoglobin: influence of structure of the globin on the heme iron. Annu Rev Biochem. 1979;48:327–386. doi: 10.1146/annurev.bi.48.070179.001551. [DOI] [PubMed] [Google Scholar]
- Rosenberg H., Modrak J. B., Hassing J. M., Al-Turk W. A., Stohs S. J. Glycosylated collagen. Biochem Biophys Res Commun. 1979 Nov 28;91(2):498–501. doi: 10.1016/0006-291x(79)91549-3. [DOI] [PubMed] [Google Scholar]
- Ryan U. S., White L. A. Varicose veins as a source of adult human endothelial cells. Tissue Cell. 1985;17(2):171–176. doi: 10.1016/0040-8166(85)90086-2. [DOI] [PubMed] [Google Scholar]
- Schleicher E., Deufel T., Wieland O. H. Non-enzymatic glycosylation of human serum lipoproteins. Elevated epsilon-lysine glycosylated low density lipoprotein in diabetic patients. FEBS Lett. 1981 Jun 29;129(1):1–4. doi: 10.1016/0014-5793(81)80741-7. [DOI] [PubMed] [Google Scholar]
- Schwartz C. J., Sprague E. A., Kelley J. L., Valente A. J., Suenram C. A. Aortic intimal monocyte recruitment in the normo and hypercholesterolemic baboon (Papio cynocephalus). An ultrastructural study: implications in atherogenesis. Virchows Arch A Pathol Anat Histopathol. 1985;405(2):175–191. doi: 10.1007/BF00704370. [DOI] [PubMed] [Google Scholar]
- Shapiro R., McManus M. J., Zalut C., Bunn H. F. Sites of nonenzymatic glycosylation of human hemoglobin A. J Biol Chem. 1980 Apr 10;255(7):3120–3127. [PubMed] [Google Scholar]
- Spatz M., Bembry J., Dodson R. F., Hervonen H., Murray M. R. Endothelial cell cultures derived from isolated cerebral microvessels. Brain Res. 1980 Jun 9;191(2):577–582. doi: 10.1016/0006-8993(80)91310-4. [DOI] [PubMed] [Google Scholar]
- Stein O., Stein Y. Bovine aortic endothelial cells display macrophage-like properties towards acetylated 125I-labelled low density lipoprotein. Biochim Biophys Acta. 1980 Dec 5;620(3):631–635. doi: 10.1016/0005-2760(80)90155-1. [DOI] [PubMed] [Google Scholar]
- Vlassara H., Brownlee M., Cerami A. Novel macrophage receptor for glucose-modified proteins is distinct from previously described scavenger receptors. J Exp Med. 1986 Oct 1;164(4):1301–1309. doi: 10.1084/jem.164.4.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther B. T., Ohman R., Roseman S. A quantitative assay for intercellular adhesion. Proc Natl Acad Sci U S A. 1973 May;70(5):1569–1573. doi: 10.1073/pnas.70.5.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams S. K., Devenny J. J., Bitensky M. W. Micropinocytic ingestion of glycosylated albumin by isolated microvessels: possible role in pathogenesis of diabetic microangiopathy. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2393–2397. doi: 10.1073/pnas.78.4.2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterhalter K. H. Non enzymatic glycosylation of proteins. Prog Clin Biol Res. 1985;195:109–122. [PubMed] [Google Scholar]
- Witztum J. L., Fisher M., Pietro T., Steinbrecher U. P., Elam R. L. Nonenzymatic glucosylation of high-density lipoprotein accelerates its catabolism in guinea pigs. Diabetes. 1982 Nov;31(11):1029–1032. doi: 10.2337/diacare.31.11.1029. [DOI] [PubMed] [Google Scholar]
- Zaman Z., Verwilghen R. L. Non-enzymic glycosylation of horse spleen and rat liver ferritins. Biochim Biophys Acta. 1981 Jul 28;669(2):120–124. doi: 10.1016/0005-2795(81)90232-4. [DOI] [PubMed] [Google Scholar]


