Abstract
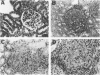
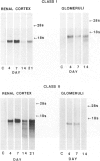
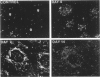
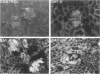
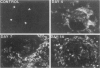
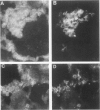
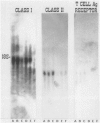
The major interacting components of the immune system, major histocompatibility complex (MHC) class I and class II proteins and T cells were analyzed in a model of anti-GBM (glomerular basement membrane) disease in the rabbit that progresses to develop cellular crescents and glomerular and interstitial fibrosis. Class I and II mRNA and protein were measured in isolated glomeruli and whole renal cortex using cDNA probes and monoclonal antibodies. The distribution of T cells and class I and II proteins was assessed by immunofluorescence. Normal glomeruli contained no T cells and were class II negative. By day 4, glomeruli contained MHC class I and II mRNA and protein and class II positive T cells. Although some animals had T cells in the periglomerular area, these cells were class II negative. By day 7 periglomerular T cells were largely class II positive (activated) and there was increased MHC class I and II mRNA and protein in whole renal cortex. Later T cells accumulated in the tubulo-interstitial compartment, which became diffusely positive for MHC classes I and II, but to a variable extent in different animals. Those with high class II mRNA expression also had detectable T cell antigen receptor mRNA by Northern analysis. The authors conclude 1) in this model there was a close association between mRNA abundance and protein expression for both MHC classes I and II in glomeruli and renal cortex as a whole; 2) in this model of glomerular injury there are three phases of activation. The first phase takes place in the glomerulus and is associated with accumulation of activated T cells and MHC class I and II protein in the glomerulus. Phase 2 is associated with the accumulation of periglomerular T cells and their becoming class II positive. There is subsequent dissemination (phase 3) of activated T cells and accumulation of class I and II mRNA and protein throughout the interstitial compartment. This spacial progression of glomerulocentric inflammation is likely associated with degree of injury and permanent loss of renal function.
Full text
PDF



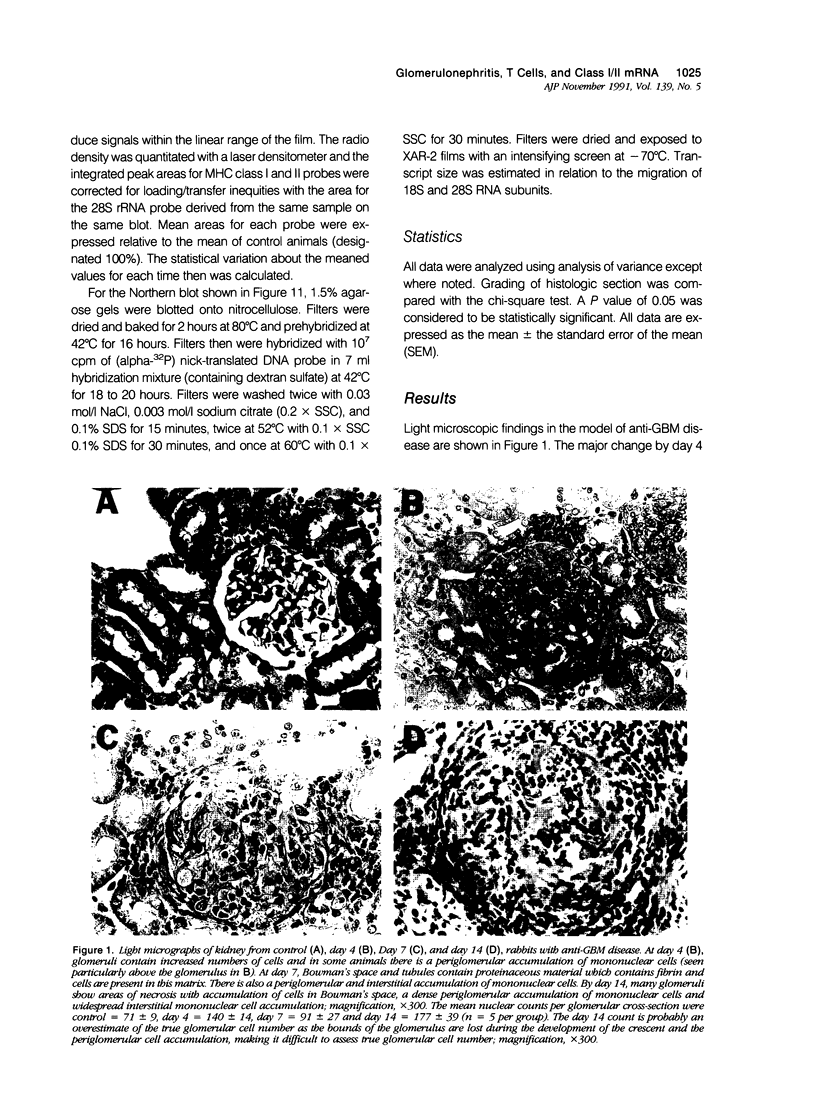
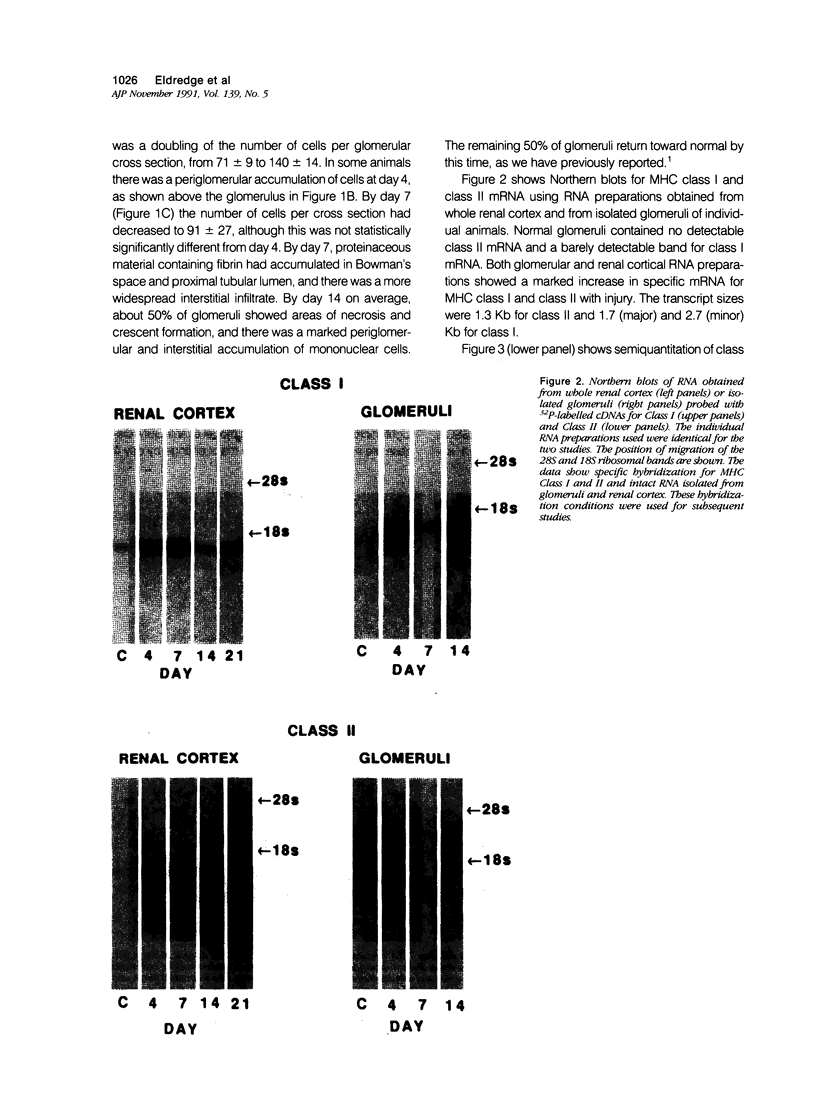
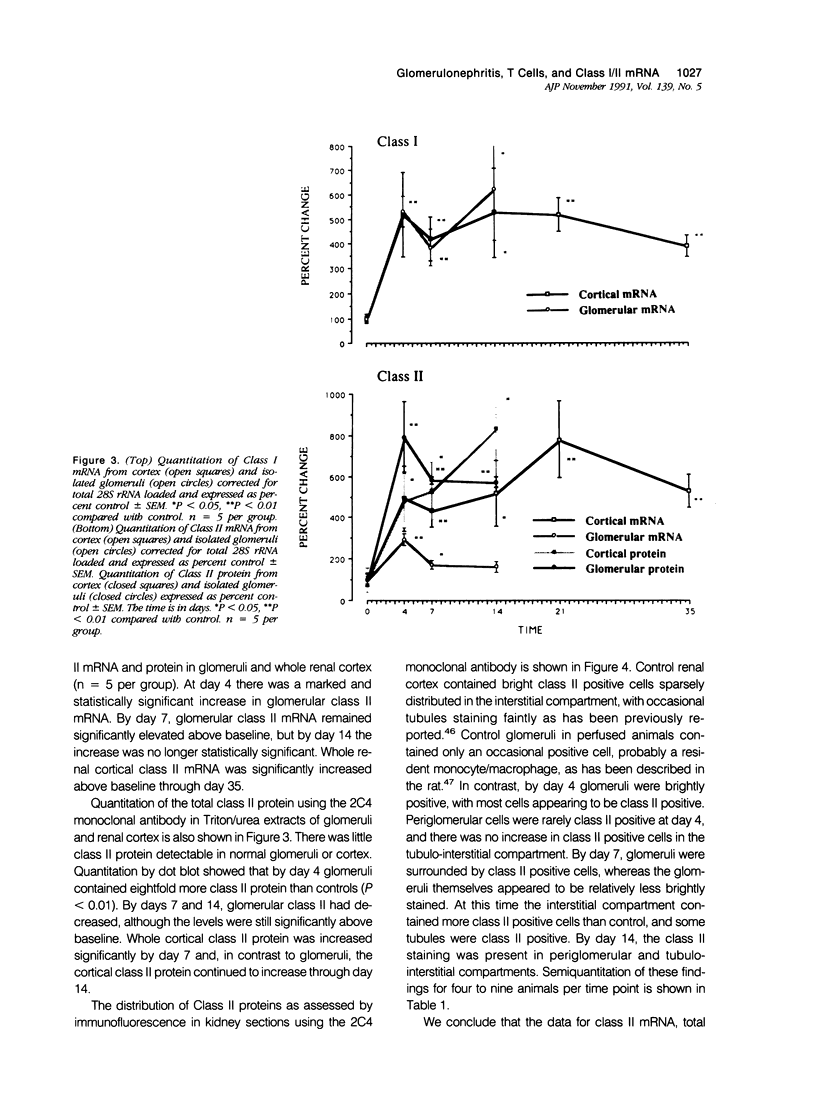
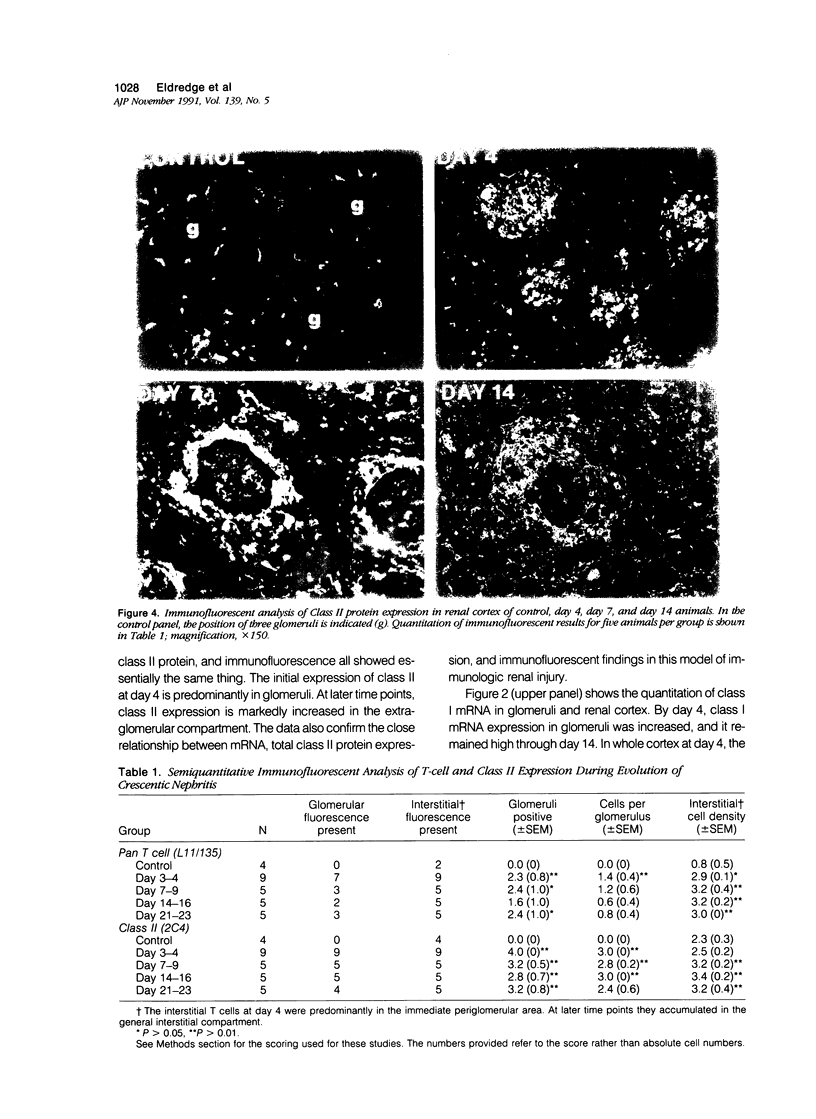

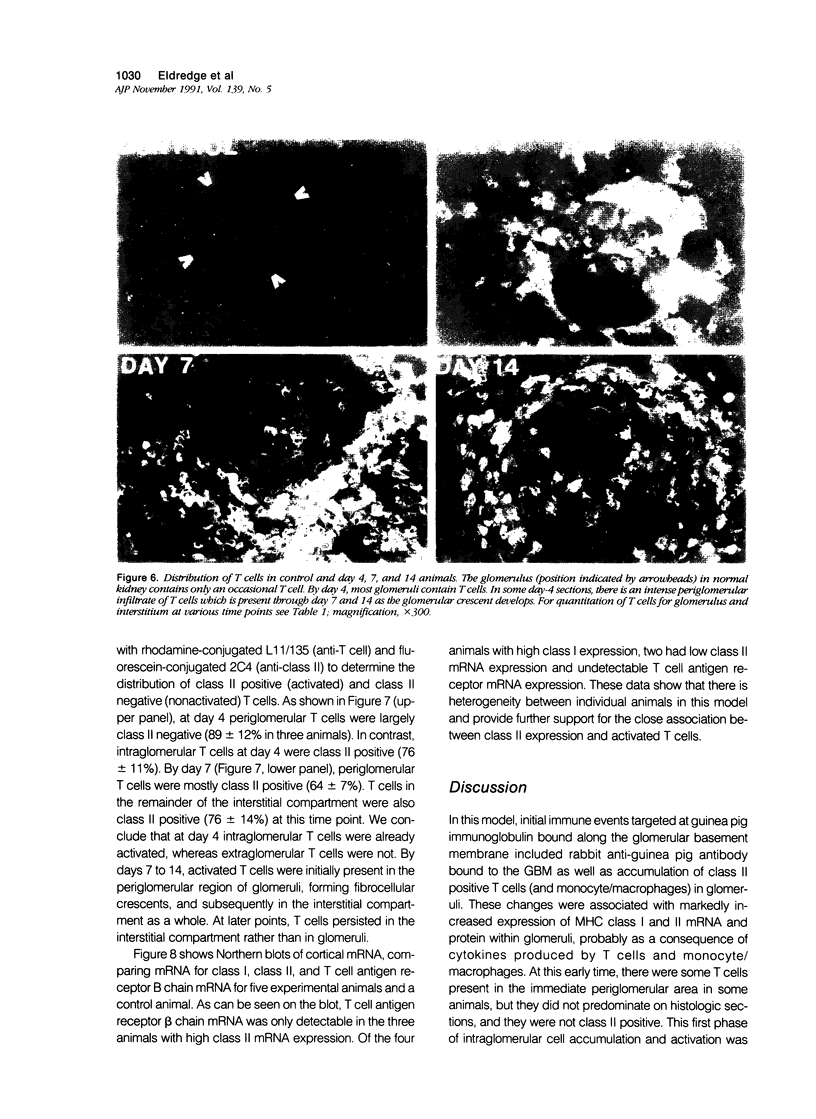





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andres G., Brentjens J., Kohli R., Anthone R., Anthone S., Baliah T., Montes M., Mookerjee B. K., Prezyna A., Sepulveda M. Histology of human tubulo-interstitial nephritis associated with antibodies to renal basement membranes. Kidney Int. 1978 Jun;13(6):480–491. doi: 10.1038/ki.1978.71. [DOI] [PubMed] [Google Scholar]
- BENOIT F. L., RULON D. B., THEIL G. B., DOOLAN P. D., WATTEN R. H. GOODPASTURE'S SYNDROME: A CLINICOPATHOLOGIC ENTITY. Am J Med. 1964 Sep;37:424–444. doi: 10.1016/0002-9343(64)90199-8. [DOI] [PubMed] [Google Scholar]
- Balow J. E., Austin H. A., 3rd, Tsokos G. C., Antonovych T. T., Steinberg A. D., Klippel J. H. NIH conference. Lupus nephritis. Ann Intern Med. 1987 Jan;106(1):79–94. doi: 10.7326/0003-4819-106-1-79. [DOI] [PubMed] [Google Scholar]
- Berg R., Bergstrand A., Bergström J., Cunningham R., Tagesson C., Wasserman J. Rapidly progressive glomerulonephritis with antibodies against glomerular basement membranes in serum and kidneys. Clin Nephrol. 1976 Jan;5(1):37–43. [PubMed] [Google Scholar]
- Bhan A. K., Collins A. B., Schneeberger E. E., McCluskey R. T. A cell-mediated reaction against glomerular-bound immune complexes. J Exp Med. 1979 Dec 1;150(6):1410–1420. doi: 10.1084/jem.150.6.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhan A. K., Schneeberger E. E., Collins A. B., McCluskey R. T. Evidence for a pathogenic role of a cell-mediated immune mechanism in experimental glomerulonephritis. J Exp Med. 1978 Jul 1;148(1):246–260. doi: 10.1084/jem.148.1.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohle A., Christ H., Grund K. E., Mackensen S. The role of the interstitium of the renal cortex in renal disease. Contrib Nephrol. 1979;16:109–114. doi: 10.1159/000402883. [DOI] [PubMed] [Google Scholar]
- Bolton W. K., Innes D. J., Jr, Sturgill B. C., Kaiser D. L. T-cells and macrophages in rapidly progressive glomerulonephritis: clinicopathologic correlations. Kidney Int. 1987 Dec;32(6):869–876. doi: 10.1038/ki.1987.288. [DOI] [PubMed] [Google Scholar]
- Bolton W. K., Tucker F. L., Sturgill B. C. New avian model of experimental glomerulonephritis consistent with mediation by cellular immunity. Nonhumorally mediated glomerulonephritis in chickens. J Clin Invest. 1984 May;73(5):1263–1276. doi: 10.1172/JCI111328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher A., Droz D., Adafer E., Noël L. H. Characterization of mononuclear cell subsets in renal cellular interstitial infiltrates. Kidney Int. 1986 May;29(5):1043–1049. doi: 10.1038/ki.1986.105. [DOI] [PubMed] [Google Scholar]
- Boucher A., Droz D., Adafer E., Noël L. H. Relationship between the integrity of Bowman's capsule and the composition of cellular crescents in human crescentic glomerulonephritis. Lab Invest. 1987 May;56(5):526–533. [PubMed] [Google Scholar]
- Brunati C., Brando B., Confalonieri R., Belli L. S., Lavagni M. G., Minetti L. Immunophenotyping of mononuclear cell infiltrates associated with renal disease. Clin Nephrol. 1986 Jul;26(1):15–20. [PubMed] [Google Scholar]
- Castiglione A., Bucci A., Fellin G., d'Amico G., Atkins R. C. The relationship of infiltrating renal leucocytes to disease activity in lupus and cryoglobulinaemic glomerulonephritis. Nephron. 1988;50(1):14–23. doi: 10.1159/000185110. [DOI] [PubMed] [Google Scholar]
- Cattell V., Jamieson S. W. The origin of glomerular crescents in experimental nephrotoxic serum nephritis in the rabbit. Lab Invest. 1978 Dec;39(6):584–590. [PubMed] [Google Scholar]
- Chan Y. L., Olvera J., Wool I. G. The structure of rat 28S ribosomal ribonucleic acid inferred from the sequence of nucleotides in a gene. Nucleic Acids Res. 1983 Nov 25;11(22):7819–7831. doi: 10.1093/nar/11.22.7819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Coimbra T., Wiggins R., Noh J. W., Merritt S., Phan S. H. Transforming growth factor-beta production in anti-glomerular basement membrane disease in the rabbit. Am J Pathol. 1991 Jan;138(1):223–234. [PMC free article] [PubMed] [Google Scholar]
- Collins T., Lapierre L. A., Fiers W., Strominger J. L., Pober J. S. Recombinant human tumor necrosis factor increases mRNA levels and surface expression of HLA-A,B antigens in vascular endothelial cells and dermal fibroblasts in vitro. Proc Natl Acad Sci U S A. 1986 Jan;83(2):446–450. doi: 10.1073/pnas.83.2.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downer G., Phan S. H., Wiggins R. C. Analysis of renal fibrosis in a rabbit model of crescentic nephritis. J Clin Invest. 1988 Sep;82(3):998–1006. doi: 10.1172/JCI113710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomini P., Tecce R., Gambari R., Sacchi A., Fisher P. B., Natali P. G. Recombinant human IFN-gamma, but not IFN-alpha or IFN-beta, enhances MHC- and non-MHC-encoded glycoproteins by a protein synthesis-dependent mechanism. J Immunol. 1988 May 1;140(9):3073–3081. [PubMed] [Google Scholar]
- Goyal M., Wiggins R. Fibronectin mRNA and protein accumulation, distribution, and breakdown in rabbit anti-glomerular basement membrane disease. J Am Soc Nephrol. 1991 Jun;1(12):1334–1342. doi: 10.1681/ASN.V1121334. [DOI] [PubMed] [Google Scholar]
- Gurner A., Smith J., Cattell V. In vivo induction of Ia antigen in resident leukocytes in the normal rat renal glomerulus. Lab Invest. 1986 Nov;55(5):546–550. [PubMed] [Google Scholar]
- Hart D. N., Fabre J. W. Endogenously produced Ia antigens within cells of convoluted tubules of rat kidney. J Immunol. 1981 Jun;126(6):2109–2113. [PubMed] [Google Scholar]
- Heilman R. L., Offord K. P., Holley K. E., Velosa J. A. Analysis of risk factors for patient and renal survival in crescentic glomerulonephritis. Am J Kidney Dis. 1987 Feb;9(2):98–107. doi: 10.1016/s0272-6386(87)80086-0. [DOI] [PubMed] [Google Scholar]
- Herrin D. L., Schmidt G. W. Rapid, reversible staining of northern blots prior to hybridization. Biotechniques. 1988 Mar;6(3):196-7, 199-200. [PubMed] [Google Scholar]
- Hooke D. H., Gee D. C., Atkins R. C. Leukocyte analysis using monoclonal antibodies in human glomerulonephritis. Kidney Int. 1987 Apr;31(4):964–972. doi: 10.1038/ki.1987.93. [DOI] [PubMed] [Google Scholar]
- Hooke D. H., Hancock W. W., Gee D. C., Kraft N., Atkins R. C. Monoclonal antibody analysis of glomerular hypercellularity in human glomerulonephritis. Clin Nephrol. 1984 Oct;22(4):163–168. [PubMed] [Google Scholar]
- Hunsicker L. G., Shearer T. P., Plattner S. B., Weisenburger D. The role of monocytes in serum sickness nephritis. J Exp Med. 1979 Sep 19;150(3):413–425. doi: 10.1084/jem.150.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson S., Sogn J. A., Kindt T. J. Microdetermination of rabbit immunoglobulin allotypes by ELISA using specific antibodies conjugated with peroxidase or with biotin. J Immunol Methods. 1982;48(3):299–309. doi: 10.1016/0022-1759(82)90331-3. [DOI] [PubMed] [Google Scholar]
- Johnson J. P., Moore J., Jr, Austin H. A., 3rd, Balow J. E., Antonovych T. T., Wilson C. B. Therapy of anti-glomerular basement membrane antibody disease: analysis of prognostic significance of clinical, pathologic and treatment factors. Medicine (Baltimore) 1985 Jul;64(4):219–227. doi: 10.1097/00005792-198507000-00003. [DOI] [PubMed] [Google Scholar]
- Kondo Y., Shigematsu H., Kobayashi Y. Cellular aspects of rabbit Masugi nephritis. II. Progressive glomerular injuries with crescent formation. Lab Invest. 1972 Dec;27(6):620–631. [PubMed] [Google Scholar]
- Kreisberg J. I., Wayne D. B., Karnovsky M. J. Rapid and focal loss of negative charge associated with mononuclear cell infiltration early in nephrotoxic serum nephritis. Kidney Int. 1979 Sep;16(3):290–300. doi: 10.1038/ki.1979.131. [DOI] [PubMed] [Google Scholar]
- Kähäri V. M., Chen Y. Q., Su M. W., Ramirez F., Uitto J. Tumor necrosis factor-alpha and interferon-gamma suppress the activation of human type I collagen gene expression by transforming growth factor-beta 1. Evidence for two distinct mechanisms of inhibition at the transcriptional and posttranscriptional levels. J Clin Invest. 1990 Nov;86(5):1489–1495. doi: 10.1172/JCI114866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laverriere A., Kulaga H., Kindt T. J., LeGuern C., Marche P. N. A rabbit class II MHC gene with strong similarities to HLA-DRA. Immunogenetics. 1989;30(2):137–140. doi: 10.1007/BF02421545. [DOI] [PubMed] [Google Scholar]
- Le Guern A., Wetterskog D., Marche P. N., Kindt T. J. A monoclonal antibody directed against a synthetic peptide reacts with a cell surface rabbit class I MHC molecule. Mol Immunol. 1987 May;24(5):455–461. doi: 10.1016/0161-5890(87)90019-8. [DOI] [PubMed] [Google Scholar]
- Leszczynski D. Interleukin-1 alpha inhibits the effects of gamma-interferon and tumor necrosis factor alpha on the expression of the major histocompatibility antigens by the rat endothelium. Am J Pathol. 1990 Jan;136(1):229–237. [PMC free article] [PubMed] [Google Scholar]
- Lobel S. A., Knight K. L. The role of rabbit Ia molecules in immune functions as determined with the use of an anti-Ia monoclonal antibody. Immunology. 1984 Jan;51(1):35–43. [PMC free article] [PubMed] [Google Scholar]
- Marche P. N., Kindt T. J. A variable region gene subfamily encoding T cell receptor beta-chains is selectively conserved among mammals. J Immunol. 1986 Sep 1;137(5):1729–1734. [PubMed] [Google Scholar]
- Martin M., Schwinzer R., Schellekens H., Resch K. Glomerular mesangial cells in local inflammation. Induction of the expression of MHC class II antigens by IFN-gamma. J Immunol. 1989 Mar 15;142(6):1887–1894. [PubMed] [Google Scholar]
- Merritt S. E., Killen P. D., Phan S. H., Wiggins R. C. Analysis of alpha 1 (I) procollagen alpha 1 (IV) collagen, and beta-actin mRNA in glomerulus and cortex of rabbits with experimental anti-glomerular basement membrane disease. Evidence for early extraglomerular collagen biosynthesis. Lab Invest. 1990 Dec;63(6):762–769. [PubMed] [Google Scholar]
- Neilson E. G., Jimenez S. A., Phillips S. M. Cell-mediated immunity in interstitial nephritis. III. T lymphocyte-mediated fibroblast proliferation and collagen synthesis: an immune mechanism for renal fibrogenesis. J Immunol. 1980 Oct;125(4):1708–1714. [PubMed] [Google Scholar]
- Neilson E. G. Pathogenesis and therapy of interstitial nephritis. Kidney Int. 1989 May;35(5):1257–1270. doi: 10.1038/ki.1989.118. [DOI] [PubMed] [Google Scholar]
- Nolasco F. E., Cameron J. S., Hartley B., Coelho A., Hildreth G., Reuben R. Intraglomerular T cells and monocytes in nephritis: study with monoclonal antibodies. Kidney Int. 1987 May;31(5):1160–1166. doi: 10.1038/ki.1987.123. [DOI] [PubMed] [Google Scholar]
- Risdon R. A., Sloper J. C., De Wardener H. E. Relationship between renal function and histological changes found in renal-biopsy specimens from patients with persistent glomerular nephritis. Lancet. 1968 Aug 17;2(7564):363–366. doi: 10.1016/s0140-6736(68)90589-8. [DOI] [PubMed] [Google Scholar]
- Sabadini E., Castiglione A., Colasanti G., Ferrario F., Civardi R., Fellin G., D'Amico G. Characterization of interstitial infiltrating cells in Berger's disease. Am J Kidney Dis. 1988 Oct;12(4):307–315. doi: 10.1016/s0272-6386(88)80225-7. [DOI] [PubMed] [Google Scholar]
- Schainuck L. I., Striker G. E., Cutler R. E., Benditt E. P. Structural-functional correlations in renal disease. II. The correlations. Hum Pathol. 1970 Dec;1(4):631–641. doi: 10.1016/s0046-8177(70)80061-2. [DOI] [PubMed] [Google Scholar]
- Schreiner G. F., Cotran R. S. Localization of an Ia-bearing glomerular cell in the mesangium. J Cell Biol. 1982 Aug;94(2):483–488. doi: 10.1083/jcb.94.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner G. F., Cotran R. S., Unanue E. R. Modulation of Ia and leukocyte common antigen expression in rat glomeruli during the course of glomerulonephritis and aminonucleoside nephrosis. Lab Invest. 1984 Nov;51(5):524–533. [PubMed] [Google Scholar]
- Stachura I., Si L., Madan E., Whiteside T. Mononuclear cell subsets in human renal disease. Enumeration in tissue sections with monoclonal antibodies. Clin Immunol Immunopathol. 1984 Mar;30(3):362–373. doi: 10.1016/0090-1229(84)90022-9. [DOI] [PubMed] [Google Scholar]
- Teague C. A., Doak P. B., Simpson I. J., Rainer S. P., Herdson P. B. Goodpasture's syndrome: an analysis of 29 cases. Kidney Int. 1978 Jun;13(6):492–504. doi: 10.1038/ki.1978.72. [DOI] [PubMed] [Google Scholar]
- Tykocinski M. L., Marche P. N., Max E. E., Kindt T. J. Rabbit class I MHC genes: cDNA clones define full-length transcripts of an expressed gene and a putative pseudogene. J Immunol. 1984 Oct;133(4):2261–2269. [PubMed] [Google Scholar]
- Unanue E. R., Dixon F. J. Experimental glomerulonephritis: immunological events and pathogenetic mechanisms. Adv Immunol. 1967;6:1–90. doi: 10.1016/s0065-2776(08)60521-0. [DOI] [PubMed] [Google Scholar]
- Wahl S. M., Hunt D. A., Wong H. L., Dougherty S., McCartney-Francis N., Wahl L. M., Ellingsworth L., Schmidt J. A., Hall G., Roberts A. B. Transforming growth factor-beta is a potent immunosuppressive agent that inhibits IL-1-dependent lymphocyte proliferation. J Immunol. 1988 May 1;140(9):3026–3032. [PubMed] [Google Scholar]
- Walker R. G., Scheinkestel C., Becker G. J., Owen J. E., Dowling J. P., Kincaid-Smith P. Clinical and morphological aspects of the management of crescentic anti-glomerular basement membrane antibody (anti-GBM) nephritis/Goodpasture's syndrome. Q J Med. 1985 Jan;54(213):75–89. [PubMed] [Google Scholar]
- Whitworth J. A., Morel-Maroger L., Mignon F., Richet G. The significance of extracapillary proliferation. Clinicopathological review of 60 patients. Nephron. 1976;16(1):1–19. doi: 10.1159/000180578. [DOI] [PubMed] [Google Scholar]
- Wiggins R. C., Glatfelter A., Brukman J. Procoagulant activity in glomeruli and urine of rabbits with nephrotoxic nephritis. Lab Invest. 1985 Aug;53(2):156–165. [PubMed] [Google Scholar]
- Wilkinson J. M., Wetterskog D. L., Sogn J. A., Kindt T. J. Cell surface glycoproteins of rabbit lymphocytes: characterization with monoclonal antibodies. Mol Immunol. 1984 Jan;21(1):95–103. doi: 10.1016/0161-5890(84)90094-4. [DOI] [PubMed] [Google Scholar]
- von Willebrand E., Lautenschlager I., Inkinen K., Lehto V. P., Virtanen I., Häyry P. Distribution of the major histocompatibility complex antigens in human and rat kidney. Kidney Int. 1985 Apr;27(4):616–621. doi: 10.1038/ki.1985.55. [DOI] [PubMed] [Google Scholar]