Abstract
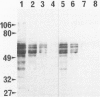
Using the hydrated autoclaving method, a new immunohistochemical procedure to enhance tau immunoreactivity in formalin-fixed brain tissue, the authors recently reported that tau protein is detected in neuronal cell bodies and proximal dendrites, gray matter neuropil, axons, and glial cells in normal human hippocampus and neocortex. In the this study, the authors performed a comparative study of the distribution of normal and modified forms of tau in Alzheimer's disease (AD) and control brains. In the cerebral cortex and white matter of AD brains, a massive accumulation of modified tau and/or severe depletion of normal tau were documented in all the tau compartments. In mild AD cases, gray matter neuropil, axons, and glial cells were less severely involved than neuronal perikarya. In the controls, neuronal perikarya were often involved by modified tau accumulation, but the other compartments showed normal distribution. These observations suggest that modifications of tau which lead to neurofibrillary lesions in AD may begin in neuronal perikarya and extend to the other tau compartments in advanced stages of the disease.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Binder L. I., Frankfurter A., Rebhun L. I. The distribution of tau in the mammalian central nervous system. J Cell Biol. 1985 Oct;101(4):1371–1378. doi: 10.1083/jcb.101.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H., Braak E., Grundke-Iqbal I., Iqbal K. Occurrence of neuropil threads in the senile human brain and in Alzheimer's disease: a third location of paired helical filaments outside of neurofibrillary tangles and neuritic plaques. Neurosci Lett. 1986 Apr 24;65(3):351–355. doi: 10.1016/0304-3940(86)90288-0. [DOI] [PubMed] [Google Scholar]
- Brion J. P., Guilleminot J., Couchie D., Flament-Durand J., Nunez J. Both adult and juvenile tau microtubule-associated proteins are axon specific in the developing and adult rat cerebellum. Neuroscience. 1988 Apr;25(1):139–146. doi: 10.1016/0306-4522(88)90013-9. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Hwo S. Y., Kirschner M. W. Purification of tau, a microtubule-associated protein that induces assembly of microtubules from purified tubulin. J Mol Biol. 1977 Oct 25;116(2):207–225. doi: 10.1016/0022-2836(77)90213-3. [DOI] [PubMed] [Google Scholar]
- Delacourte A., Flament S., Dibe E. M., Hublau P., Sablonnière B., Hémon B., Shérrer V., Défossez A. Pathological proteins Tau 64 and 69 are specifically expressed in the somatodendritic domain of the degenerating cortical neurons during Alzheimer's disease. Demonstration with a panel of antibodies against Tau proteins. Acta Neuropathol. 1990;80(2):111–117. doi: 10.1007/BF00308912. [DOI] [PubMed] [Google Scholar]
- Flament S., Delacourte A. Abnormal tau species are produced during Alzheimer's disease neurodegenerating process. FEBS Lett. 1989 Apr 24;247(2):213–216. doi: 10.1016/0014-5793(89)81337-7. [DOI] [PubMed] [Google Scholar]
- Grundke-Iqbal I., Iqbal K., Tung Y. C., Quinlan M., Wisniewski H. M., Binder L. I. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihara Y., Abraham C., Selkoe D. J. Antibodies to paired helical filaments in Alzheimer's disease do not recognize normal brain proteins. Nature. 1983 Aug 25;304(5928):727–730. doi: 10.1038/304727a0. [DOI] [PubMed] [Google Scholar]
- Ihara Y. Massive somatodendritic sprouting of cortical neurons in Alzheimer's disease. Brain Res. 1988 Aug 30;459(1):138–144. doi: 10.1016/0006-8993(88)90293-4. [DOI] [PubMed] [Google Scholar]
- Ihara Y., Nukina N., Miura R., Ogawara M. Phosphorylated tau protein is integrated into paired helical filaments in Alzheimer's disease. J Biochem. 1986 Jun;99(6):1807–1810. doi: 10.1093/oxfordjournals.jbchem.a135662. [DOI] [PubMed] [Google Scholar]
- Iqbal K., Grundke-Iqbal I., Zaidi T., Merz P. A., Wen G. Y., Shaikh S. S., Wisniewski H. M., Alafuzoff I., Winblad B. Defective brain microtubule assembly in Alzheimer's disease. Lancet. 1986 Aug 23;2(8504):421–426. doi: 10.1016/s0140-6736(86)92134-3. [DOI] [PubMed] [Google Scholar]
- Khachaturian Z. S. Diagnosis of Alzheimer's disease. Arch Neurol. 1985 Nov;42(11):1097–1105. doi: 10.1001/archneur.1985.04060100083029. [DOI] [PubMed] [Google Scholar]
- Kosik K. S., Crandall J. E., Mufson E. J., Neve R. L. Tau in situ hybridization in normal and Alzheimer brain: localization in the somatodendritic compartment. Ann Neurol. 1989 Sep;26(3):352–361. doi: 10.1002/ana.410260308. [DOI] [PubMed] [Google Scholar]
- Kosik K. S., Finch E. A. MAP2 and tau segregate into dendritic and axonal domains after the elaboration of morphologically distinct neurites: an immunocytochemical study of cultured rat cerebrum. J Neurosci. 1987 Oct;7(10):3142–3153. doi: 10.1523/JNEUROSCI.07-10-03142.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosik K. S., Orecchio L. D., Binder L., Trojanowski J. Q., Lee V. M., Lee G. Epitopes that span the tau molecule are shared with paired helical filaments. Neuron. 1988 Nov;1(9):817–825. doi: 10.1016/0896-6273(88)90129-8. [DOI] [PubMed] [Google Scholar]
- Kowall N. W., Kosik K. S. Axonal disruption and aberrant localization of tau protein characterize the neuropil pathology of Alzheimer's disease. Ann Neurol. 1987 Nov;22(5):639–643. doi: 10.1002/ana.410220514. [DOI] [PubMed] [Google Scholar]
- Ksiezak-Reding H., Binder L. I., Yen S. H. Immunochemical and biochemical characterization of tau proteins in normal and Alzheimer's disease brains with Alz 50 and Tau-1. J Biol Chem. 1988 Jun 15;263(17):7948–7953. [PubMed] [Google Scholar]
- Ksiezak-Reding H., Davies P., Yen S. H. Alz 50, a monoclonal antibody to Alzheimer's disease antigen, cross-reacts with tau proteins from bovine and normal human brain. J Biol Chem. 1988 Jun 15;263(17):7943–7947. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee V. M., Balin B. J., Otvos L., Jr, Trojanowski J. Q. A68: a major subunit of paired helical filaments and derivatized forms of normal Tau. Science. 1991 Feb 8;251(4994):675–678. doi: 10.1126/science.1899488. [DOI] [PubMed] [Google Scholar]
- Migheli A., Butler M., Brown K., Shelanski M. L. Light and electron microscope localization of the microtubule-associated tau protein in rat brain. J Neurosci. 1988 Jun;8(6):1846–1851. doi: 10.1523/JNEUROSCI.08-06-01846.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nukina N., Kosik K. S., Selkoe D. J. The monoclonal antibody, Alz 50, recognizes tau proteins in Alzheimer's disease brain. Neurosci Lett. 1988 May 3;87(3):240–246. doi: 10.1016/0304-3940(88)90455-7. [DOI] [PubMed] [Google Scholar]
- Papasozomenos S. C., Binder L. I. Phosphorylation determines two distinct species of Tau in the central nervous system. Cell Motil Cytoskeleton. 1987;8(3):210–226. doi: 10.1002/cm.970080303. [DOI] [PubMed] [Google Scholar]
- Papasozomenos S. C. Tau protein immunoreactivity in dementia of the Alzheimer type. I. Morphology, evolution, distribution, and pathogenetic implications. Lab Invest. 1989 Jan;60(1):123–137. [PubMed] [Google Scholar]
- Pollock N. J., Wood J. G. Differential sensitivity of the microtubule-associated protein, tau, in Alzheimer's disease tissue to formalin fixation. J Histochem Cytochem. 1988 Sep;36(9):1117–1121. doi: 10.1177/36.9.2841371. [DOI] [PubMed] [Google Scholar]
- Schmidt M. L., Lee V. M., Trojanowski J. Q. Comparative epitope analysis of neuronal cytoskeletal proteins in Alzheimer's disease senile plaque neurites and neuropil threads. Lab Invest. 1991 Mar;64(3):352–357. [PubMed] [Google Scholar]
- Shin R. W., Iwaki T., Kitamoto T., Tateishi J. Hydrated autoclave pretreatment enhances tau immunoreactivity in formalin-fixed normal and Alzheimer's disease brain tissues. Lab Invest. 1991 May;64(5):693–702. [PubMed] [Google Scholar]
- Shin R. W., Kitamoto T., Tateishi J. Modified tau is present in younger nondemented persons: a study of subcortical nuclei in Alzheimer's disease and progressive supranuclear palsy. Acta Neuropathol. 1991;81(5):517–523. doi: 10.1007/BF00310132. [DOI] [PubMed] [Google Scholar]
- Shin R. W., Ogomori K., Kitamoto T., Tateishi J. Increased tau accumulation in senile plaques as a hallmark in Alzheimer's disease. Am J Pathol. 1989 Jun;134(6):1365–1371. [PMC free article] [PubMed] [Google Scholar]
- Sternberger L. A., Sternberger N. H. Monoclonal antibodies distinguish phosphorylated and nonphosphorylated forms of neurofilaments in situ. Proc Natl Acad Sci U S A. 1983 Oct;80(19):6126–6130. doi: 10.1073/pnas.80.19.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trojanowski J. Q., Schuck T., Schmidt M. L., Lee V. M. Distribution of tau proteins in the normal human central and peripheral nervous system. J Histochem Cytochem. 1989 Feb;37(2):209–215. doi: 10.1177/37.2.2492045. [DOI] [PubMed] [Google Scholar]
- Weingarten M. D., Lockwood A. H., Hwo S. Y., Kirschner M. W. A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A. 1975 May;72(5):1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolozin B. L., Pruchnicki A., Dickson D. W., Davies P. A neuronal antigen in the brains of Alzheimer patients. Science. 1986 May 2;232(4750):648–650. doi: 10.1126/science.3083509. [DOI] [PubMed] [Google Scholar]
- Wolozin B., Scicutella A., Davies P. Reexpression of a developmentally regulated antigen in Down syndrome and Alzheimer disease. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6202–6206. doi: 10.1073/pnas.85.16.6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood J. G., Mirra S. S., Pollock N. J., Binder L. I. Neurofibrillary tangles of Alzheimer disease share antigenic determinants with the axonal microtubule-associated protein tau (tau) Proc Natl Acad Sci U S A. 1986 Jun;83(11):4040–4043. doi: 10.1073/pnas.83.11.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T., McGeer P. L. Oligodendroglial microtubular masses: an abnormality observed in some human neurodegenerative diseases. Neurosci Lett. 1990 Dec 11;120(2):163–166. doi: 10.1016/0304-3940(90)90028-8. [DOI] [PubMed] [Google Scholar]