Abstract
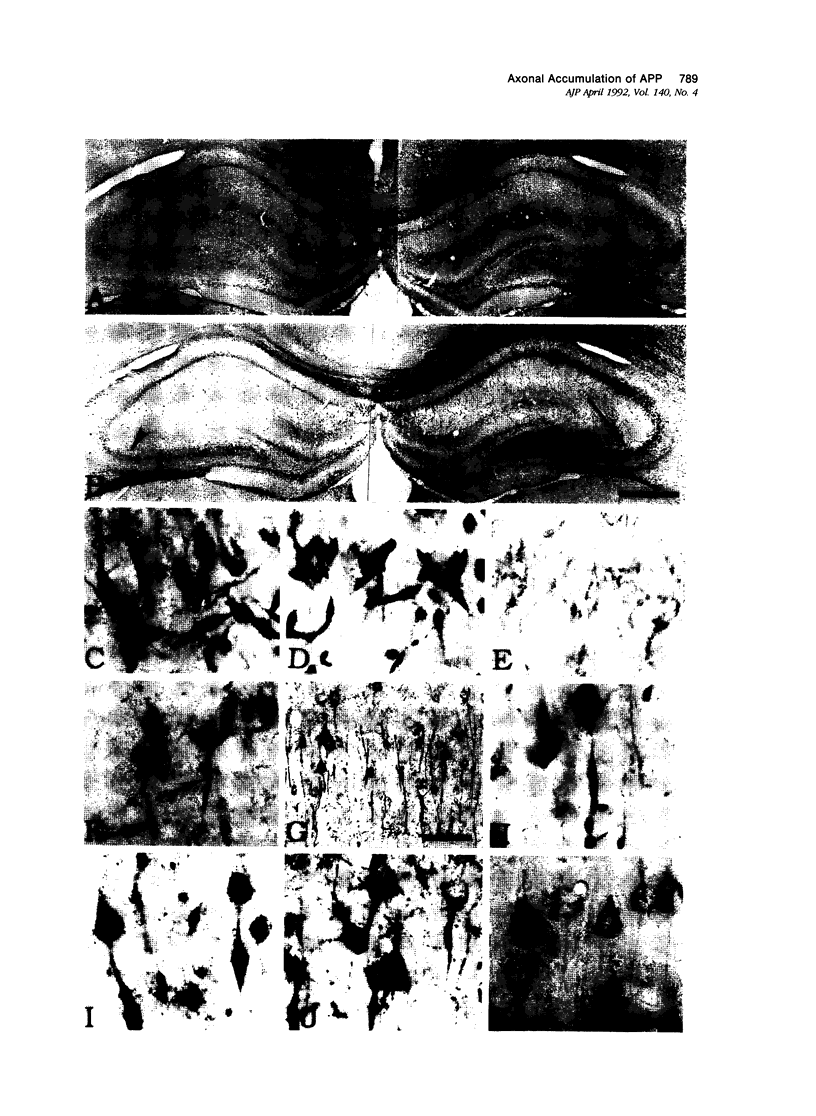
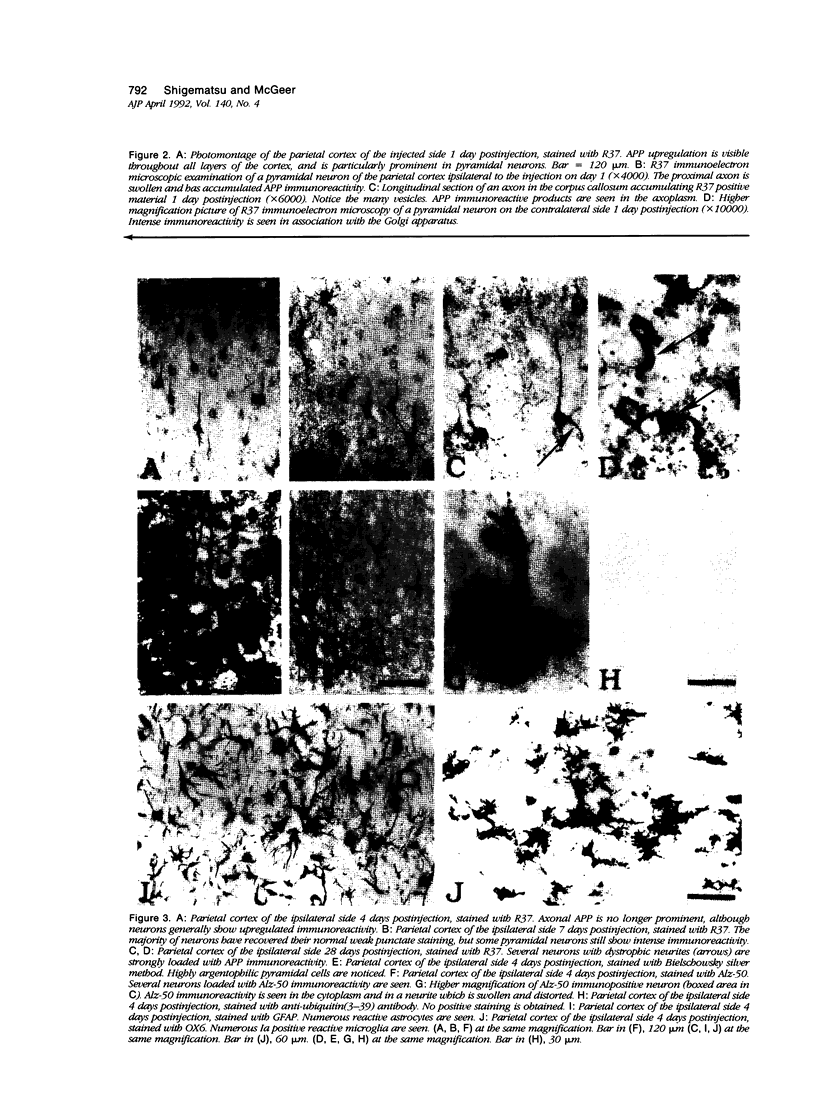
To study a possible relationship between inhibition of axonal flow and amyloidogenesis, the authors examined amyloid precursor protein (APP) immunoreactivity in rat brain treated with colchicine. After intraventricular injection of colchicine, the proximal axons of exposed neurons became swollen and showed a large increase in APP immunoreactivity, whereas the cytoplasm and dendritic processes showed lesser increases. These changes were seen in ipsilateral neurons of the hippocampus, lateral septal nucleus, amygdala, and entorhinal, parietal and temporal cortices, as well as bilaterally in the periventricular hypothalamic nucleus. The increase of APP immunoreactivity appeared as early as 3 hours after the injection. It peaked at around 24 hours, and began to clear after about 4 days. A few strongly APP-positive dystrophic neurons remained. In serial sections at these later time periods, some strongly argentophilic neurons and Alz-50 positive neurons, each with abnormal neurities, could be demonstrated. The result suggests that APP may undergo fast axoplasmic flow in rat brain and that argentophilic changes of Alz-50 immunoproduction may follow APP accumulation caused by inhibition of axoplasmic flow.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berkenbosch F., van Oers J., del Rey A., Tilders F., Besedovsky H. Corticotropin-releasing factor-producing neurons in the rat activated by interleukin-1. Science. 1987 Oct 23;238(4826):524–526. doi: 10.1126/science.2443979. [DOI] [PubMed] [Google Scholar]
- Cole G. M., Masliah E., Shelton E. R., Chan H. W., Terry R. D., Saitoh T. Accumulation of amyloid precursor fragment in Alzheimer plaques. Neurobiol Aging. 1991 Mar-Apr;12(2):85–91. doi: 10.1016/0197-4580(91)90046-m. [DOI] [PubMed] [Google Scholar]
- Emerich D. F., Walsh T. J. Cholinergic cell loss and cognitive impairments following intraventricular or intradentate injection of colchicine. Brain Res. 1990 May 28;517(1-2):157–167. doi: 10.1016/0006-8993(90)91021-8. [DOI] [PubMed] [Google Scholar]
- Gajdusek D. C. Hypothesis: interference with axonal transport of neurofilament as a common pathogenetic mechanism in certain diseases of the central nervous system. N Engl J Med. 1985 Mar 14;312(11):714–719. doi: 10.1056/NEJM198503143121110. [DOI] [PubMed] [Google Scholar]
- Goedert M., Spillantini M. G., Jakes R. Localization of the Alz-50 epitope in recombinant human microtubule-associated protein tau. Neurosci Lett. 1991 May 27;126(2):149–154. doi: 10.1016/0304-3940(91)90541-z. [DOI] [PubMed] [Google Scholar]
- Goldschmidt R. B., Steward O. Preferential neurotoxicity of colchicine for granule cells of the dentate gyrus of the adult rat. Proc Natl Acad Sci U S A. 1980 May;77(5):3047–3051. doi: 10.1073/pnas.77.5.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorenstein C. A hypothesis concerning the role of endogenous colchicine-like factors in the etiology of Alzheimer's disease. Med Hypotheses. 1987 Aug;23(4):371–374. doi: 10.1016/0306-9877(87)90057-0. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Hyman B. T., Van Hoesen G. W., Wolozin B. L., Davies P., Kromer L. J., Damasio A. R. Alz-50 antibody recognizes Alzheimer-related neuronal changes. Ann Neurol. 1988 Apr;23(4):371–379. doi: 10.1002/ana.410230410. [DOI] [PubMed] [Google Scholar]
- Ishii T., Kametani F., Haga S., Sato M. The immunohistochemical demonstration of subsequences of the precursor of the amyloid A4 protein in senile plaques in Alzheimer's disease. Neuropathol Appl Neurobiol. 1989 Mar-Apr;15(2):135–147. doi: 10.1111/j.1365-2990.1989.tb01216.x. [DOI] [PubMed] [Google Scholar]
- Johnstone E. M., Chaney M. O., Norris F. H., Pascual R., Little S. P. Conservation of the sequence of the Alzheimer's disease amyloid peptide in dog, polar bear and five other mammals by cross-species polymerase chain reaction analysis. Brain Res Mol Brain Res. 1991 Jul;10(4):299–305. doi: 10.1016/0169-328x(91)90088-f. [DOI] [PubMed] [Google Scholar]
- KLATZO I., WISNIEWSKI H., STREICHER E. EXPERIMENTAL PRODUCTION OF NEUROFIBRILLARY DEGENERATION. I. LIGHT MICROSCOPIC OBSERVATIONS. J Neuropathol Exp Neurol. 1965 Apr;24:187–199. doi: 10.1097/00005072-196504000-00002. [DOI] [PubMed] [Google Scholar]
- Kametani F., Haga S., Tanaka K., Ishii T. Amyloid beta-protein precursor (APP) of cultured cells: secretory and non-secretory forms of APP. J Neurol Sci. 1990 Jun;97(1):43–52. doi: 10.1016/0022-510x(90)90097-7. [DOI] [PubMed] [Google Scholar]
- Kang J., Lemaire H. G., Unterbeck A., Salbaum J. M., Masters C. L., Grzeschik K. H., Multhaup G., Beyreuther K., Müller-Hill B. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987 Feb 19;325(6106):733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Kawabata S., Higgins G. A., Gordon J. W. Amyloid plaques, neurofibrillary tangles and neuronal loss in brains of transgenic mice overexpressing a C-terminal fragment of human amyloid precursor protein. Nature. 1991 Dec 12;354(6353):476–478. doi: 10.1038/354476a0. [DOI] [PubMed] [Google Scholar]
- Koo E. H., Sisodia S. S., Archer D. R., Martin L. J., Weidemann A., Beyreuther K., Fischer P., Masters C. L., Price D. L. Precursor of amyloid protein in Alzheimer disease undergoes fast anterograde axonal transport. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1561–1565. doi: 10.1073/pnas.87.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosik K. S., Joachim C. L., Selkoe D. J. Microtubule-associated protein tau (tau) is a major antigenic component of paired helical filaments in Alzheimer disease. Proc Natl Acad Sci U S A. 1986 Jun;83(11):4044–4048. doi: 10.1073/pnas.83.11.4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowall N. W., Beal M. F., Busciglio J., Duffy L. K., Yankner B. A. An in vivo model for the neurodegenerative effects of beta amyloid and protection by substance P. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7247–7251. doi: 10.1073/pnas.88.16.7247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee V. M., Balin B. J., Otvos L., Jr, Trojanowski J. Q. A68: a major subunit of paired helical filaments and derivatized forms of normal Tau. Science. 1991 Feb 8;251(4994):675–678. doi: 10.1126/science.1899488. [DOI] [PubMed] [Google Scholar]
- McGeer P. L., Akiyama H., Kawamata T., Yamada T., Walker D. G., Ishii T. Immunohistochemical localization of beta-amyloid precursor protein sequences in Alzheimer and normal brain tissue by light and electron microscopy. J Neurosci Res. 1992 Mar;31(3):428–442. doi: 10.1002/jnr.490310305. [DOI] [PubMed] [Google Scholar]
- McGeer P. L., Itagaki S., Tago H., McGeer E. G. Reactive microglia in patients with senile dementia of the Alzheimer type are positive for the histocompatibility glycoprotein HLA-DR. Neurosci Lett. 1987 Aug 18;79(1-2):195–200. doi: 10.1016/0304-3940(87)90696-3. [DOI] [PubMed] [Google Scholar]
- McMaster W. R., Williams A. F. Identification of Ia glycoproteins in rat thymus and purification from rat spleen. Eur J Immunol. 1979 Jun;9(6):426–433. doi: 10.1002/eji.1830090603. [DOI] [PubMed] [Google Scholar]
- Quon D., Wang Y., Catalano R., Scardina J. M., Murakami K., Cordell B. Formation of beta-amyloid protein deposits in brains of transgenic mice. Nature. 1991 Jul 18;352(6332):239–241. doi: 10.1038/352239a0. [DOI] [PubMed] [Google Scholar]
- Refolo L. M., Wittenberg I. S., Friedrich V. L., Jr, Robakis N. K. The Alzheimer amyloid precursor is associated with the detergent-insoluble cytoskeleton. J Neurosci. 1991 Dec;11(12):3888–3897. doi: 10.1523/JNEUROSCI.11-12-03888.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigematsu K., McGeer P. L., Walker D. G., Ishii T., McGeer E. G. Reactive microglia/macrophages phagocytose amyloid precursor protein produced by neurons following neural damage. J Neurosci Res. 1992 Mar;31(3):443–453. doi: 10.1002/jnr.490310306. [DOI] [PubMed] [Google Scholar]
- Shivers B. D., Hilbich C., Multhaup G., Salbaum M., Beyreuther K., Seeburg P. H. Alzheimer's disease amyloidogenic glycoprotein: expression pattern in rat brain suggests a role in cell contact. EMBO J. 1988 May;7(5):1365–1370. doi: 10.1002/j.1460-2075.1988.tb02952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solitare G. B., Lamarche J. B. Alzheimer's disease and senile dementia as seen in mongoloids: neuropathological observations. Am J Ment Defic. 1966 May;70(6):840–848. [PubMed] [Google Scholar]
- Suzuki K., Terry R. D. Fine structural localization of acid phosphatase in senile plaques in Alzheimer's presenile dementia. Acta Neuropathol. 1967 May 5;8(3):276–284. doi: 10.1007/BF00688828. [DOI] [PubMed] [Google Scholar]
- Wang G. P., Grundke-Iqbal I., Kascsak R. J., Iqbal K., Wisniewski H. M. Alzheimer neurofibrillary tangles: monoclonal antibodies to inherent antigen(s). Acta Neuropathol. 1984;62(4):268–275. doi: 10.1007/BF00687608. [DOI] [PubMed] [Google Scholar]
- Wirak D. O., Bayney R., Ramabhadran T. V., Fracasso R. P., Hart J. T., Hauer P. E., Hsiau P., Pekar S. K., Scangos G. A., Trapp B. D. Deposits of amyloid beta protein in the central nervous system of transgenic mice. Science. 1991 Jul 19;253(5017):323–325. doi: 10.1126/science.1857970. [DOI] [PubMed] [Google Scholar]
- Wiśniewski H., Terry R. D. Experimental colchicine encephalopathy. I. Induction of neurofibrillary degeneration. Lab Invest. 1967 Dec;17(6):577–587. [PubMed] [Google Scholar]
- Wolozin B. L., Pruchnicki A., Dickson D. W., Davies P. A neuronal antigen in the brains of Alzheimer patients. Science. 1986 May 2;232(4750):648–650. doi: 10.1126/science.3083509. [DOI] [PubMed] [Google Scholar]
- Yankner B. A., Dawes L. R., Fisher S., Villa-Komaroff L., Oster-Granite M. L., Neve R. L. Neurotoxicity of a fragment of the amyloid precursor associated with Alzheimer's disease. Science. 1989 Jul 28;245(4916):417–420. doi: 10.1126/science.2474201. [DOI] [PubMed] [Google Scholar]