Abstract
The authors have compared the reactivity of monoclonal antibodies (MAb) directed against endothelial adhesion molecules (ICAM-1, ICAM-2, VCAM-1, ELAM-1) in hyperplastic, nonmalignant, and malignant lymph nodes. The authors demonstrate that the reactivity with ICAM-1 MAb is stronger in the high endothelial venules (HEV) and other smaller vessels (SV) in lymphomas compared with hyperplastic lymph nodes. Similarly, the reactivity of an ICAM-2 MAb (6D5) was shown to be higher in malignant lymph nodes compared with nonmalignant ones. ICAM-2 MAb stained both the HEV and SV. VCAM-1 MAb reacted strongly with germinal centers and its endothelial reactivity was higher in the lymphoma nodes. ELAM-1 MAb stained only faintly some endothelial cells in malignant tissue. These data suggest that besides the known regulatable endothelial adhesion molecules ICAM-1 and VCAM-1, the expression of ICAM-2 can be modified.
Full text
PDF

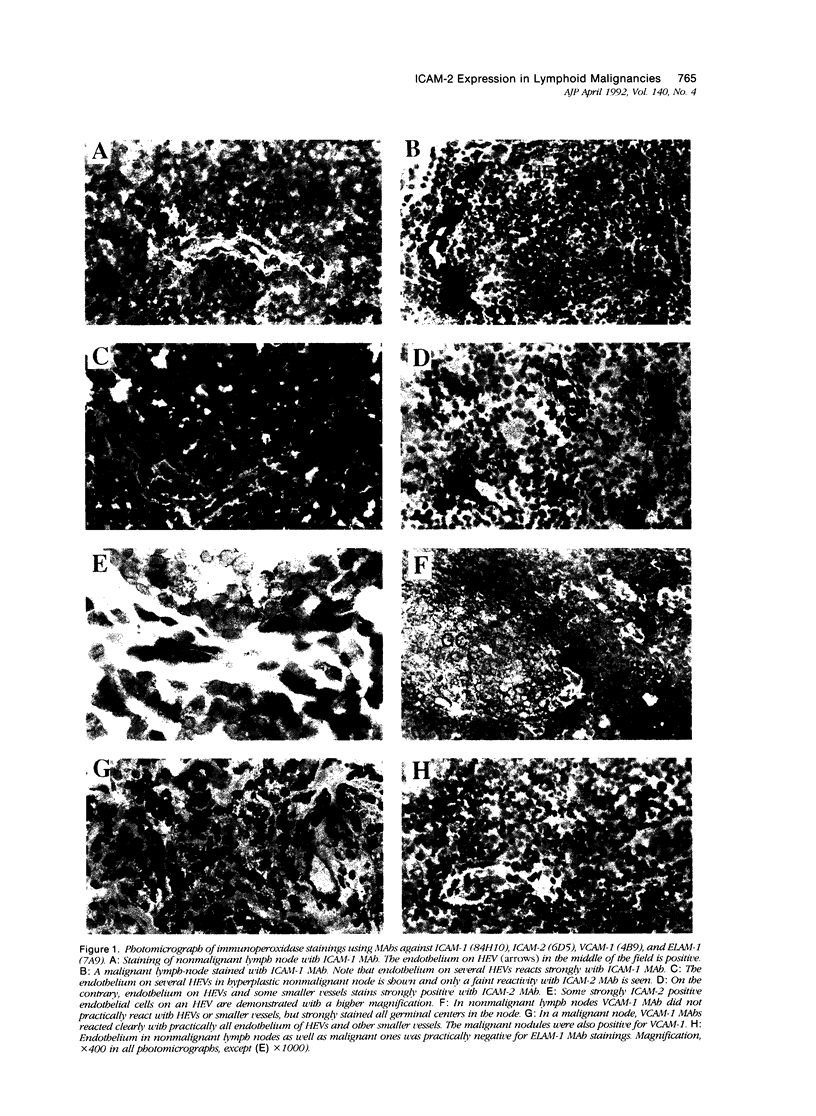
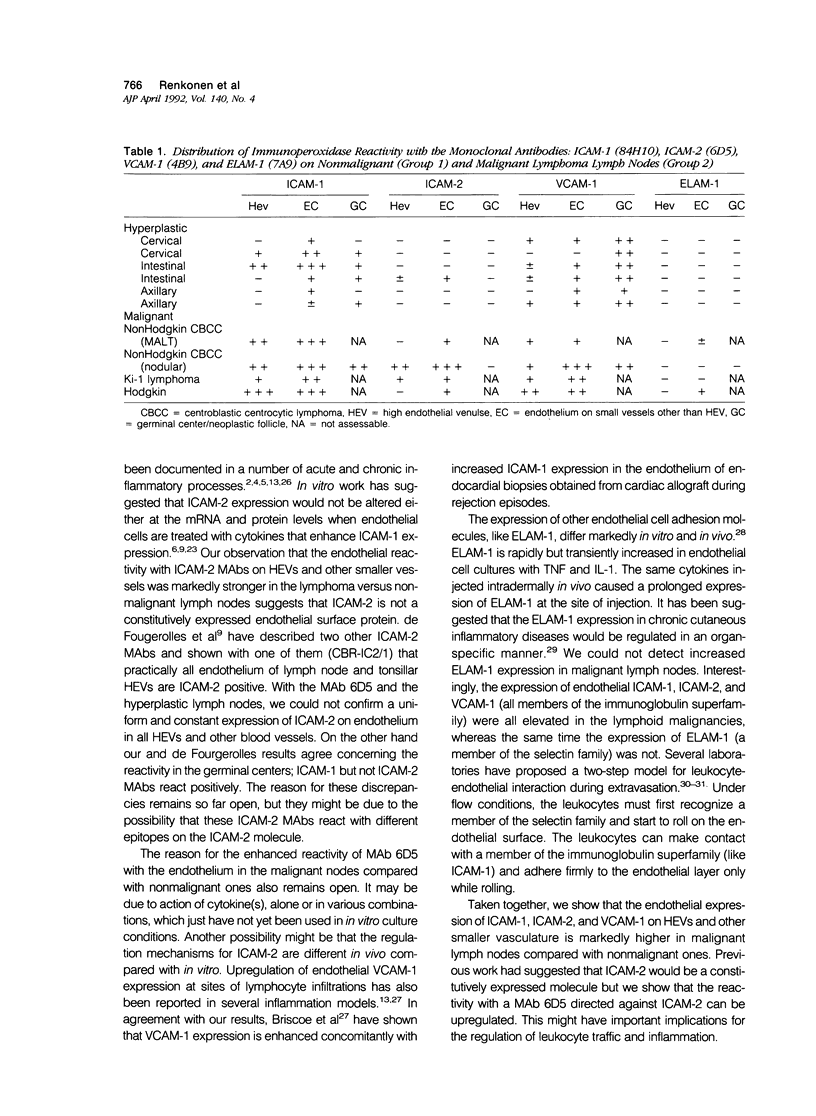

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allavena P., Paganin C., Martin-Padura I., Peri G., Gaboli M., Dejana E., Marchisio P. C., Mantovani A. Molecules and structures involved in the adhesion of natural killer cells to vascular endothelium. J Exp Med. 1991 Feb 1;173(2):439–448. doi: 10.1084/jem.173.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg E. L., Robinson M. K., Mansson O., Butcher E. C., Magnani J. L. A carbohydrate domain common to both sialyl Le(a) and sialyl Le(X) is recognized by the endothelial cell leukocyte adhesion molecule ELAM-1. J Biol Chem. 1991 Aug 15;266(23):14869–14872. [PubMed] [Google Scholar]
- Bevilacqua M. P., Stengelin S., Gimbrone M. A., Jr, Seed B. Endothelial leukocyte adhesion molecule 1: an inducible receptor for neutrophils related to complement regulatory proteins and lectins. Science. 1989 Mar 3;243(4895):1160–1165. doi: 10.1126/science.2466335. [DOI] [PubMed] [Google Scholar]
- Bochner B. S., Luscinskas F. W., Gimbrone M. A., Jr, Newman W., Sterbinsky S. A., Derse-Anthony C. P., Klunk D., Schleimer R. P. Adhesion of human basophils, eosinophils, and neutrophils to interleukin 1-activated human vascular endothelial cells: contributions of endothelial cell adhesion molecules. J Exp Med. 1991 Jun 1;173(6):1553–1557. doi: 10.1084/jem.173.6.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandley B. K., Swiedler S. J., Robbins P. W. Carbohydrate ligands of the LEC cell adhesion molecules. Cell. 1990 Nov 30;63(5):861–863. doi: 10.1016/0092-8674(90)90487-y. [DOI] [PubMed] [Google Scholar]
- Briscoe D. M., Schoen F. J., Rice G. E., Bevilacqua M. P., Ganz P., Pober J. S. Induced expression of endothelial-leukocyte adhesion molecules in human cardiac allografts. Transplantation. 1991 Feb;51(2):537–539. [PubMed] [Google Scholar]
- Butcher E. C. Warner-Lambert/Parke-Davis Award lecture. Cellular and molecular mechanisms that direct leukocyte traffic. Am J Pathol. 1990 Jan;136(1):3–11. [PMC free article] [PubMed] [Google Scholar]
- Carlos T. M., Harlan J. M. Membrane proteins involved in phagocyte adherence to endothelium. Immunol Rev. 1990 Apr;114:5–28. doi: 10.1111/j.1600-065x.1990.tb00559.x. [DOI] [PubMed] [Google Scholar]
- Diamond M. S., Staunton D. E., Marlin S. D., Springer T. A. Binding of the integrin Mac-1 (CD11b/CD18) to the third immunoglobulin-like domain of ICAM-1 (CD54) and its regulation by glycosylation. Cell. 1991 Jun 14;65(6):961–971. doi: 10.1016/0092-8674(91)90548-d. [DOI] [PubMed] [Google Scholar]
- Duijvestijn A., Hamann A. Mechanisms and regulation of lymphocyte migration. Immunol Today. 1989 Jan;10(1):23–28. doi: 10.1016/0167-5699(89)90061-3. [DOI] [PubMed] [Google Scholar]
- Elices M. J., Osborn L., Takada Y., Crouse C., Luhowskyj S., Hemler M. E., Lobb R. R. VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA-4/fibronectin binding site. Cell. 1990 Feb 23;60(4):577–584. doi: 10.1016/0092-8674(90)90661-w. [DOI] [PubMed] [Google Scholar]
- GOWANS J. L., KNIGHT E. J. THE ROUTE OF RE-CIRCULATION OF LYMPHOCYTES IN THE RAT. Proc R Soc Lond B Biol Sci. 1964 Jan 14;159:257–282. doi: 10.1098/rspb.1964.0001. [DOI] [PubMed] [Google Scholar]
- Gahmberg C. G., Nortamo P., Zimmermann D., Ruoslahti E. The human leukocyte-adhesion ligand, intercellular-adhesion molecule 2. Expression and characterization of the protein. Eur J Biochem. 1991 Jan 1;195(1):177–182. doi: 10.1111/j.1432-1033.1991.tb15692.x. [DOI] [PubMed] [Google Scholar]
- Graber N., Gopal T. V., Wilson D., Beall L. D., Polte T., Newman W. T cells bind to cytokine-activated endothelial cells via a novel, inducible sialoglycoprotein and endothelial leukocyte adhesion molecule-1. J Immunol. 1990 Aug 1;145(3):819–830. [PubMed] [Google Scholar]
- Lawrence M. B., Springer T. A. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell. 1991 May 31;65(5):859–873. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- Lobb R. R., Chi-Rosso G., Leone D. R., Rosa M. D., Bixler S., Newman B. M., Luhowskyj S., Benjamin C. D., Dougas I. G., Goelz S. E. Expression and functional characterization of a soluble form of endothelial-leukocyte adhesion molecule 1. J Immunol. 1991 Jul 1;147(1):124–129. [PubMed] [Google Scholar]
- Munro J. M., Pober J. S., Cotran R. S. Tumor necrosis factor and interferon-gamma induce distinct patterns of endothelial activation and associated leukocyte accumulation in skin of Papio anubis. Am J Pathol. 1989 Jul;135(1):121–133. [PMC free article] [PubMed] [Google Scholar]
- Nortamo P., Li R., Renkonen R., Timonen T., Prieto J., Patarroyo M., Gahmberg C. G. The expression of human intercellular adhesion molecule-2 is refractory to inflammatory cytokines. Eur J Immunol. 1991 Oct;21(10):2629–2632. doi: 10.1002/eji.1830211049. [DOI] [PubMed] [Google Scholar]
- Nortamo P., Salcedo R., Timonen T., Patarroyo M., Gahmberg C. G. A monoclonal antibody to the human leukocyte adhesion molecule intercellular adhesion molecule-2. Cellular distribution and molecular characterization of the antigen. J Immunol. 1991 Apr 15;146(8):2530–2535. [PubMed] [Google Scholar]
- Osborn L., Hession C., Tizard R., Vassallo C., Luhowskyj S., Chi-Rosso G., Lobb R. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell. 1989 Dec 22;59(6):1203–1211. doi: 10.1016/0092-8674(89)90775-7. [DOI] [PubMed] [Google Scholar]
- Osborn L. Leukocyte adhesion to endothelium in inflammation. Cell. 1990 Jul 13;62(1):3–6. doi: 10.1016/0092-8674(90)90230-c. [DOI] [PubMed] [Google Scholar]
- Patarroyo M. Leukocyte adhesion in host defense and tissue injury. Clin Immunol Immunopathol. 1991 Sep;60(3):333–348. doi: 10.1016/0090-1229(91)90091-n. [DOI] [PubMed] [Google Scholar]
- Picker L. J., Kishimoto T. K., Smith C. W., Warnock R. A., Butcher E. C. ELAM-1 is an adhesion molecule for skin-homing T cells. Nature. 1991 Feb 28;349(6312):796–799. doi: 10.1038/349796a0. [DOI] [PubMed] [Google Scholar]
- Pober J. S., Cotran R. S. The role of endothelial cells in inflammation. Transplantation. 1990 Oct;50(4):537–544. doi: 10.1097/00007890-199010000-00001. [DOI] [PubMed] [Google Scholar]
- Polley M. J., Phillips M. L., Wayner E., Nudelman E., Singhal A. K., Hakomori S., Paulson J. C. CD62 and endothelial cell-leukocyte adhesion molecule 1 (ELAM-1) recognize the same carbohydrate ligand, sialyl-Lewis x. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6224–6228. doi: 10.1073/pnas.88.14.6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu Y., Shaw S., Graber N., Gopal T. V., Horgan K. J., Van Seventer G. A., Newman W. Activation-independent binding of human memory T cells to adhesion molecule ELAM-1. Nature. 1991 Feb 28;349(6312):799–802. doi: 10.1038/349799a0. [DOI] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Springer T. A., Lasky L. A. Cell adhesion. Sticky sugars for selectins. Nature. 1991 Jan 17;349(6306):196–197. doi: 10.1038/349196a0. [DOI] [PubMed] [Google Scholar]
- Staunton D. E., Dustin M. L., Springer T. A. Functional cloning of ICAM-2, a cell adhesion ligand for LFA-1 homologous to ICAM-1. Nature. 1989 May 4;339(6219):61–64. doi: 10.1038/339061a0. [DOI] [PubMed] [Google Scholar]
- de Fougerolles A. R., Stacker S. A., Schwarting R., Springer T. A. Characterization of ICAM-2 and evidence for a third counter-receptor for LFA-1. J Exp Med. 1991 Jul 1;174(1):253–267. doi: 10.1084/jem.174.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Andrian U. H., Chambers J. D., McEvoy L. M., Bargatze R. F., Arfors K. E., Butcher E. C. Two-step model of leukocyte-endothelial cell interaction in inflammation: distinct roles for LECAM-1 and the leukocyte beta 2 integrins in vivo. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7538–7542. doi: 10.1073/pnas.88.17.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]



