Abstract
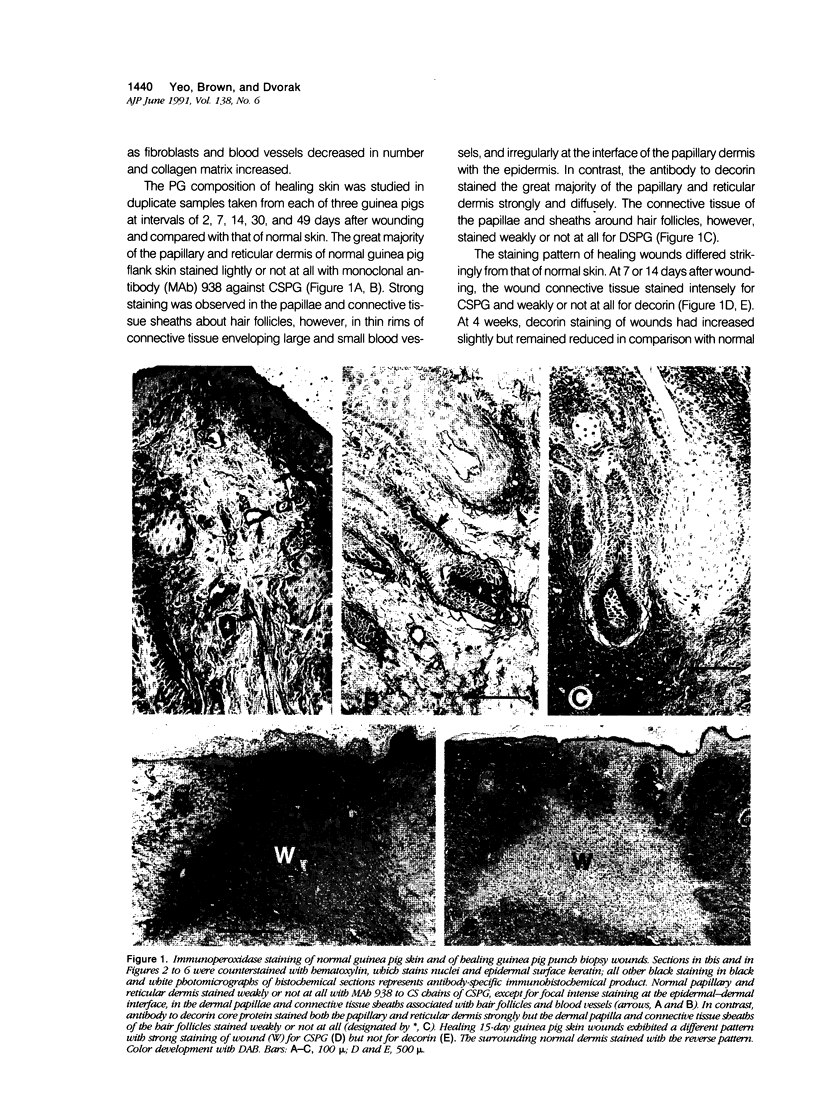
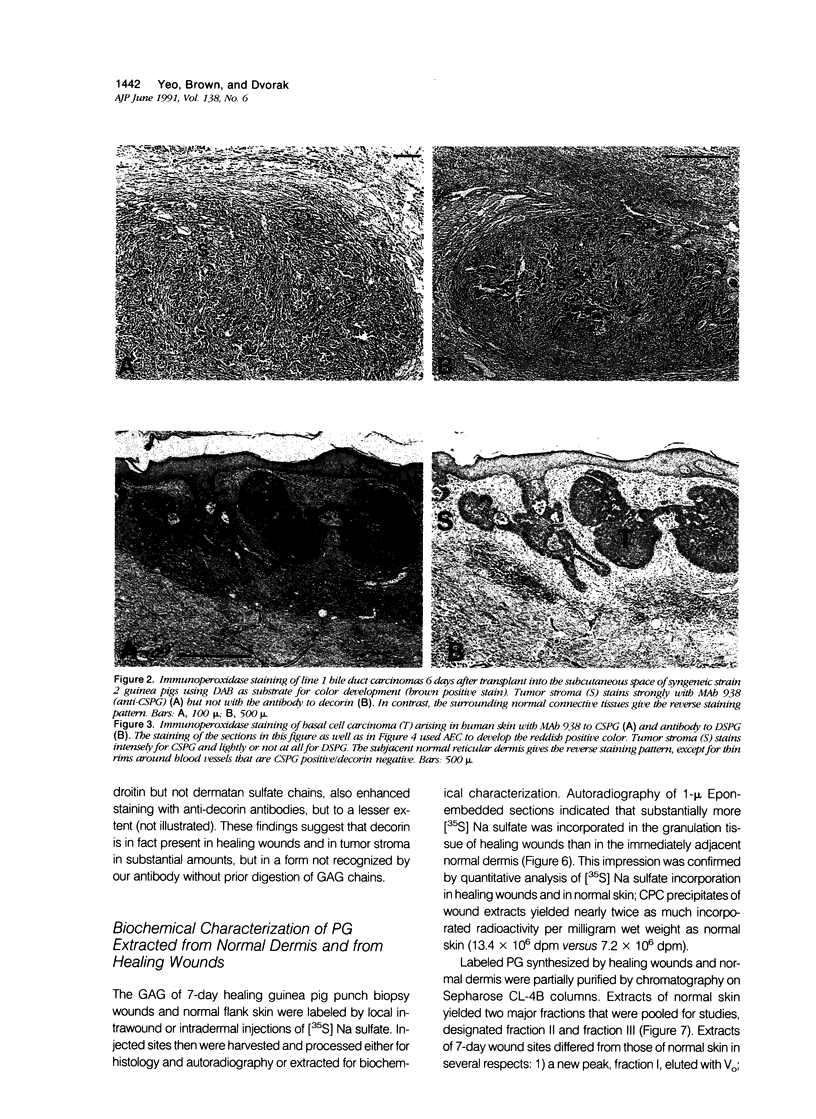
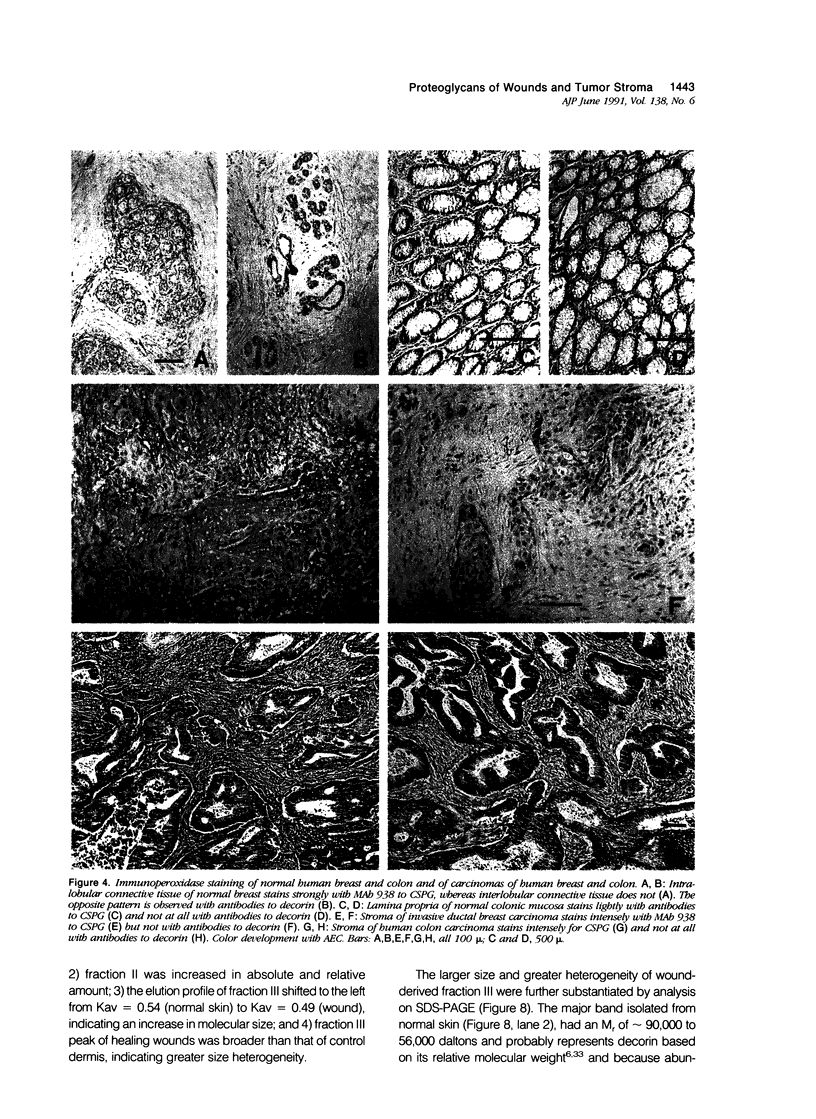
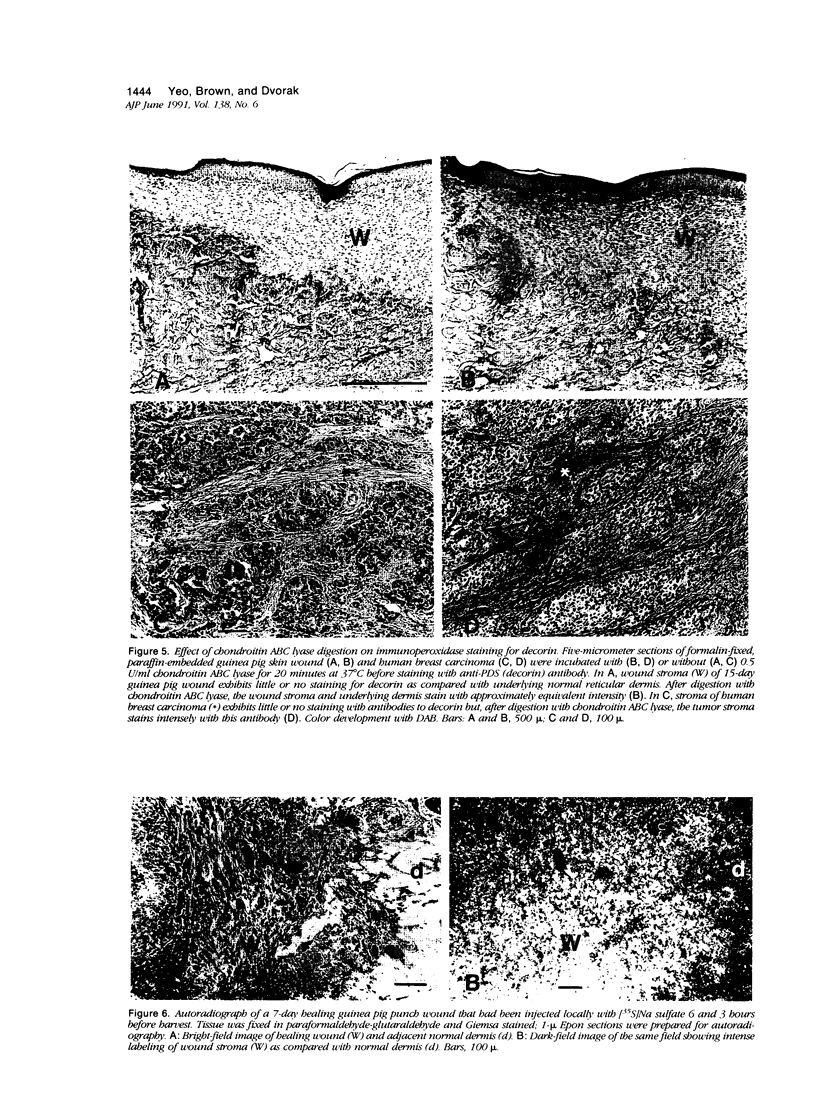
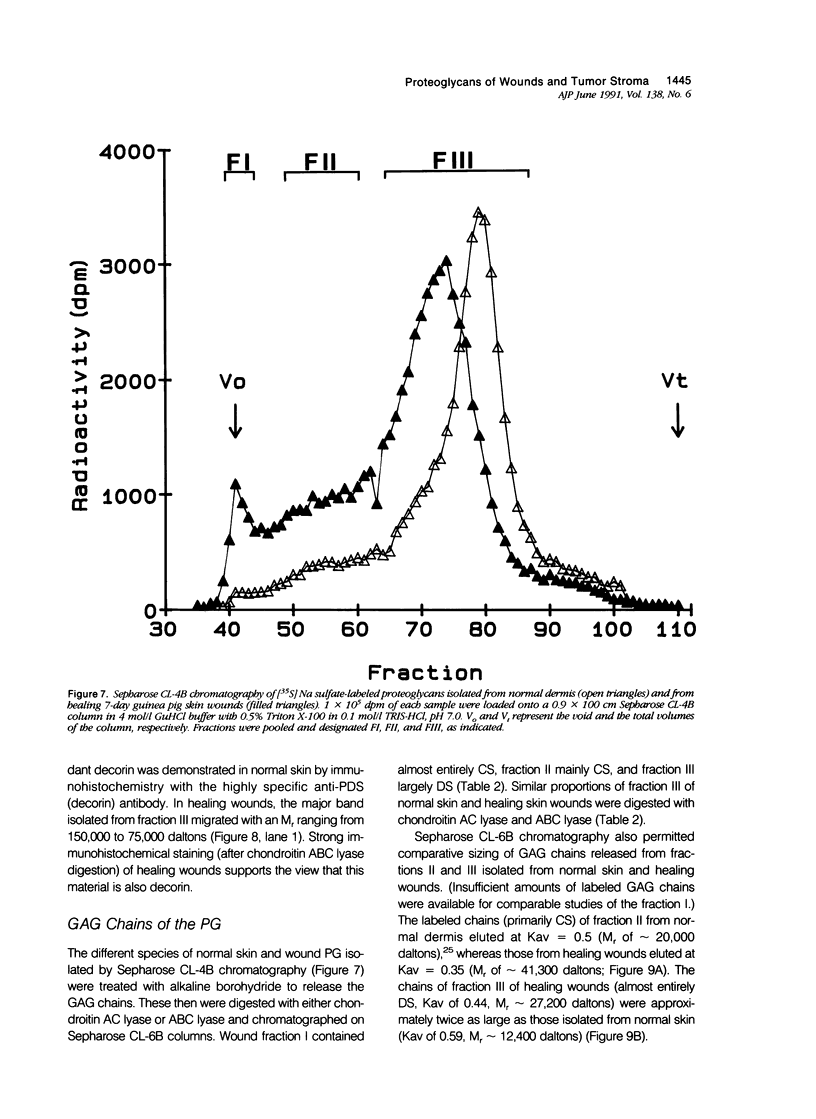
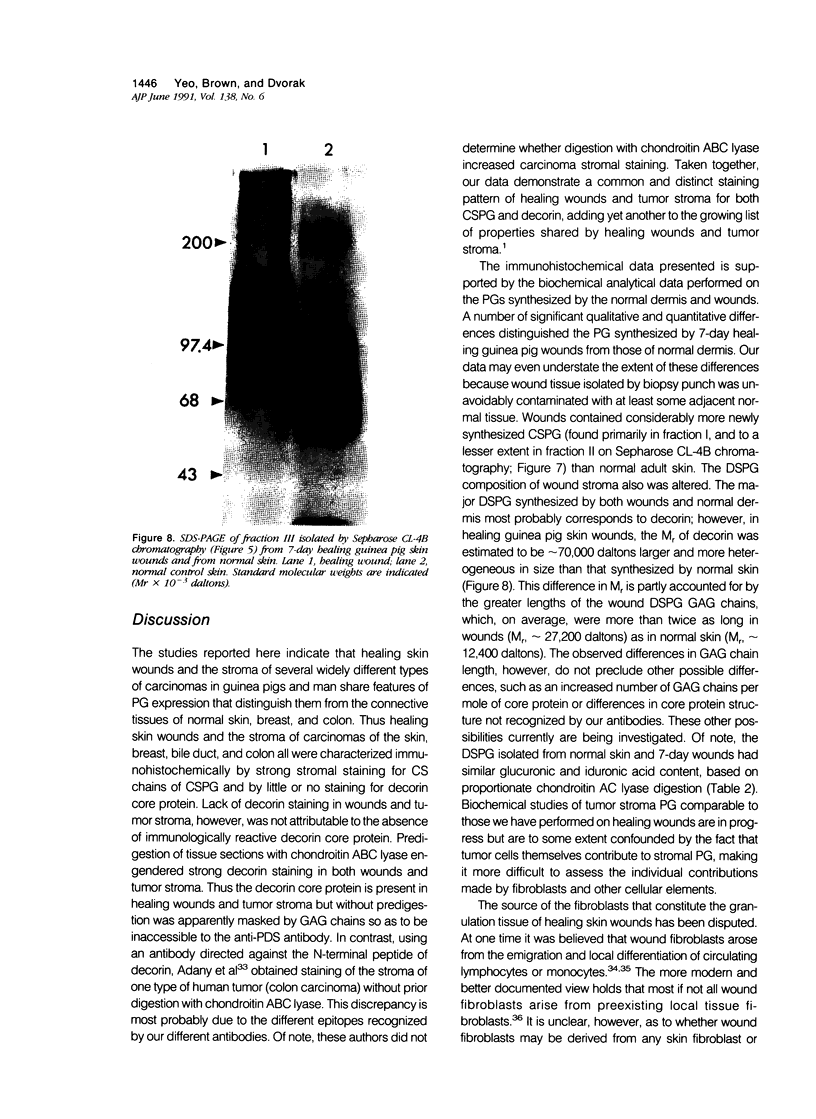
Wound healing and tumor stroma generation share several important properties, including hyperpermeable blood vessels, extravasation of fibrinogen, and extravascular clotting. In both, the deposits of fibrin gel serve initially as provisional stroma and later are replaced by granulation tissue. Proteoglycans (PG) are also important constituents of the extracellular matrix, but their composition and role in healing wounds and tumor stroma generation are poorly understood. The authors used immunohistochemical and biochemical methods to investigate the dermatan sulfate proteoglycan (DSPG) and chondroitin sulfate proteoglycan (CSPG) composition of healing skin wounds and solid tumors. By immunohistochemistry, the great majority of normal guinea pig and human dermis stained weakly for CSPG and strongly for decorin. In contrast, the granulation tissue of healing skin wounds and scars stained intensely for CSPG and weakly or not at all for decorin; however decorin staining was restored to normal intensity after digestion with chondroitin ABC lyase, suggesting that decorin antigenic sites had been masked by glycosaminoglycan (GAG) chains. Like wounds, the stroma of several carcinomas (line 1 guinea pig, human breast, colon, basal cell, and squamous) stained strongly for CSPG and weakly or not at all for decorin, but decorin staining developed after chondroitin ABC lyase digestion. Thus healing wounds and tumor stroma express a common pattern of altered PG staining, adding another to the properties these pathologic entities share. Proteoglycans extracted from healing wounds after in situ labelling with [35S] Na sulfate contained more CSPG than normal dermis with significantly longer GAG chains. Granulation tissue also synthesized more DSPG than normal skin, with greater heterogeneity and longer GAG chains. These alterations in PG synthesis correlate with the cell proliferation, migration, and collagen synthesis that accompany wound healing and may provide clues to the mechanisms responsible for both wound healing and tumor stroma generation.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adany R., Heimer R., Caterson B., Sorrell J. M., Iozzo R. V. Altered expression of chondroitin sulfate proteoglycan in the stroma of human colon carcinoma. Hypomethylation of PG-40 gene correlates with increased PG-40 content and mRNA levels. J Biol Chem. 1990 Jul 5;265(19):11389–11396. [PubMed] [Google Scholar]
- Alexander S. A., Donoff R. B. The glycosaminoglycans of open wounds. J Surg Res. 1980 Nov;29(5):422–429. doi: 10.1016/0022-4804(80)90055-4. [DOI] [PubMed] [Google Scholar]
- Bassols A., Massagué J. Transforming growth factor beta regulates the expression and structure of extracellular matrix chondroitin/dermatan sulfate proteoglycans. J Biol Chem. 1988 Feb 25;263(6):3039–3045. [PubMed] [Google Scholar]
- Chen J. K., Hoshi H., McKeehan W. L. Transforming growth factor type beta specifically stimulates synthesis of proteoglycan in human adult arterial smooth muscle cells. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5287–5291. doi: 10.1073/pnas.84.15.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cöster L., Carlstedt I., Kendall S., Malmström A., Schmidtchen A., Fransson L. A. Structure of proteoheparan sulfates from fibroblasts. Confluent and proliferating fibroblasts produce at least three types of proteoheparan sulfates with functionally different core proteins. J Biol Chem. 1986 Sep 15;261(26):12079–12088. [PubMed] [Google Scholar]
- Dodd R. M., Sigel B., Dunn M. R. Localization of new cell formation in tendon healing by tritiated thymidine autoradiography. Surg Gynecol Obstet. 1966 Apr;122(4):805–806. [PubMed] [Google Scholar]
- Dvorak H. F., Form D. M., Manseau E. J., Smith B. D. Pathogenesis of desmoplasia. I. Immunofluorescence identification and localization of some structural proteins of line 1 and line 10 guinea pig tumors and of healing wounds. J Natl Cancer Inst. 1984 Nov;73(5):1195–1205. [PubMed] [Google Scholar]
- Dvorak H. F. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986 Dec 25;315(26):1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- Esko J. D., Rostand K. S., Weinke J. L. Tumor formation dependent on proteoglycan biosynthesis. Science. 1988 Aug 26;241(4869):1092–1096. doi: 10.1126/science.3137658. [DOI] [PubMed] [Google Scholar]
- Form D. M., VanDeWater L., Dvorak H. F., Smith B. D. Pathogenesis of tumor desmoplasia. II. Collagens synthesized by line 1 and line 10 guinea pig carcinoma cells and by syngeneic fibroblasts in vitro. J Natl Cancer Inst. 1984 Nov;73(5):1207–1214. [PubMed] [Google Scholar]
- Garg A. K., Berg R. A., Silver F. H., Garg H. G. Effect of proteoglycans on type I collagen fibre formation. Biomaterials. 1989 Aug;10(6):413–419. doi: 10.1016/0142-9612(89)90133-6. [DOI] [PubMed] [Google Scholar]
- Gillman T., Wright L. J. Autoradiographic evidence suggesting in vivo transformation of some blood mononuclears in repair and fibrosis. Nature. 1966 Mar 12;209(5028):1086–1090. doi: 10.1038/2091086a0. [DOI] [PubMed] [Google Scholar]
- Glössl J., Beck M., Kresse H. Biosynthesis of proteodermatan sulfate in cultured human fibroblasts. J Biol Chem. 1984 Nov 25;259(22):14144–14150. [PubMed] [Google Scholar]
- Hamati H. F., Britton E. L., Carey D. J. Inhibition of proteoglycan synthesis alters extracellular matrix deposition, proliferation, and cytoskeletal organization of rat aortic smooth muscle cells in culture. J Cell Biol. 1989 Jun;108(6):2495–2505. doi: 10.1083/jcb.108.6.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iozzo R. V. Proteoglycans and neoplasia. Cancer Metastasis Rev. 1988 Apr;7(1):39–50. doi: 10.1007/BF00048277. [DOI] [PubMed] [Google Scholar]
- Kinsella M. G., Wight T. N. Modulation of sulfated proteoglycan synthesis by bovine aortic endothelial cells during migration. J Cell Biol. 1986 Mar;102(3):679–687. doi: 10.1083/jcb.102.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob T. J., Vogel K. G. Proteoglycan synthesis in organ cultures from regions of bovine tendon subjected to different mechanical forces. Biochem J. 1987 Sep 15;246(3):589–598. doi: 10.1042/bj2460589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krusius T., Gehlsen K. R., Ruoslahti E. A fibroblast chondroitin sulfate proteoglycan core protein contains lectin-like and growth factor-like sequences. J Biol Chem. 1987 Sep 25;262(27):13120–13125. [PubMed] [Google Scholar]
- Krusius T., Ruoslahti E. Primary structure of an extracellular matrix proteoglycan core protein deduced from cloned cDNA. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7683–7687. doi: 10.1073/pnas.83.20.7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lark M. W., Culp L. A. Multiple classes of heparan sulfate proteoglycans from fibroblast substratum adhesion sites. Affinity fractionation on columns of platelet factor 4, plasma fibronectin, and octyl-sepharose. J Biol Chem. 1984 Jun 10;259(11):6773–6782. [PubMed] [Google Scholar]
- Lark M. W., Wight T. N. Modulation of proteoglycan metabolism by aortic smooth muscle cells grown on collagen gels. Arteriosclerosis. 1986 Nov-Dec;6(6):638–650. doi: 10.1161/01.atv.6.6.638. [DOI] [PubMed] [Google Scholar]
- Lark M. W., Yeo T. K., Mar H., Lara S., Hellström I., Hellström K. E., Wight T. N. Arterial chondroitin sulfate proteoglycan: localization with a monoclonal antibody. J Histochem Cytochem. 1988 Oct;36(10):1211–1221. doi: 10.1177/36.10.3047228. [DOI] [PubMed] [Google Scholar]
- MACDONALD R. A. Origin of fibroblasts in experimental healing wounds: autoradiographic studies using tritiated thymidine. Surgery. 1959 Aug;46(2):376–382. [PubMed] [Google Scholar]
- Morales T. I., Roberts A. B. Transforming growth factor beta regulates the metabolism of proteoglycans in bovine cartilage organ cultures. J Biol Chem. 1988 Sep 15;263(26):12828–12831. [PubMed] [Google Scholar]
- Orenstein N. S., Galli S. J., Dvorak A. M., Silbert J. E., Dvorak H. F. Sulfated glycosaminoglycans of guinea pig basophilic leukocytes. J Immunol. 1978 Aug;121(2):586–592. [PubMed] [Google Scholar]
- Rosenberg L. C., Choi H. U., Tang L. H., Johnson T. L., Pal S., Webber C., Reiner A., Poole A. R. Isolation of dermatan sulfate proteoglycans from mature bovine articular cartilages. J Biol Chem. 1985 May 25;260(10):6304–6313. [PubMed] [Google Scholar]
- Ross R., Everett N. B., Tyler R. Wound healing and collagen formation. VI. The origin of the wound fibroblast studied in parabiosis. J Cell Biol. 1970 Mar;44(3):645–654. doi: 10.1083/jcb.44.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H., Yamagata T., Suzuki S. Enzymatic methods for the determination of small quantities of isomeric chondroitin sulfates. J Biol Chem. 1968 Apr 10;243(7):1536–1542. [PubMed] [Google Scholar]
- Schmidt G., Robenek H., Harrach B., Glössl J., Nolte V., Hörmann H., Richter H., Kresse H. Interaction of small dermatan sulfate proteoglycan from fibroblasts with fibronectin. J Cell Biol. 1987 Jun;104(6):1683–1691. doi: 10.1083/jcb.104.6.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. E. Collagen--proteoglycan interactions. Localization of proteoglycans in tendon by electron microscopy. Biochem J. 1980 Jun 1;187(3):887–891. doi: 10.1042/bj1870887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. E., Orford C. R. Dermatan sulphate-rich proteoglycan associates with rat tail-tendon collagen at the d band in the gap region. Biochem J. 1981 Jul 1;197(1):213–216. doi: 10.1042/bj1970213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieweke M. H., Thompson N. L., Sporn M. B., Bissell M. J. Mediation of wound-related Rous sarcoma virus tumorigenesis by TGF-beta. Science. 1990 Jun 29;248(4963):1656–1660. doi: 10.1126/science.2163544. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Autocrine growth factors and cancer. 1985 Feb 28-Mar 6Nature. 313(6005):745–747. doi: 10.1038/313745a0. [DOI] [PubMed] [Google Scholar]
- Sumrall A. J., Johnson W. C. The origin of dermal fibrocytes in wound repair. Dermatologica. 1973;146(2):107–114. doi: 10.1159/000252033. [DOI] [PubMed] [Google Scholar]
- Takeuchi J., Sobue M., Sato E., Shamoto M., Miura K. Variation in glycosaminoglycan components of breast tumors. Cancer Res. 1976 Jul;36(7 Pt 1):2133–2139. [PubMed] [Google Scholar]
- Uldbjerg N., Danielsen C. C. A study of the interaction in vitro between type I collagen and a small dermatan sulphate proteoglycan. Biochem J. 1988 May 1;251(3):643–648. doi: 10.1042/bj2510643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel K. G., Paulsson M., Heinegård D. Specific inhibition of type I and type II collagen fibrillogenesis by the small proteoglycan of tendon. Biochem J. 1984 Nov 1;223(3):587–597. doi: 10.1042/bj2230587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss B., Glössl J., Cully Z., Kresse H. Immunocytochemical investigation on the distribution of small chondroitin sulfate-dermatan sulfate proteoglycan in the human. J Histochem Cytochem. 1986 Aug;34(8):1013–1019. doi: 10.1177/34.8.2426331. [DOI] [PubMed] [Google Scholar]
- Wasteson A. A method for the determination of the molecular weight and molecular-weight distribution of chondroitin sulphate. J Chromatogr. 1971 Jul 8;59(1):87–97. doi: 10.1016/s0021-9673(01)80009-1. [DOI] [PubMed] [Google Scholar]
- Wasteson A., Uthne K., Westermark B. A novel assay for the biosynthesis of sulphated polysaccharide and its application to studies on the effects of somatomedin on cultured cells. Biochem J. 1973 Dec;136(4):1069–1074. doi: 10.1042/bj1361069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods A., Hök M., Kjellén L., Smith C. G., Rees D. A. Relationship of heparan sulfate proteoglycans to the cytoskeleton and extracellular matrix of cultured fibroblasts. J Cell Biol. 1984 Nov;99(5):1743–1753. doi: 10.1083/jcb.99.5.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y., Ruoslahti E. Expression of human proteoglycan in Chinese hamster ovary cells inhibits cell proliferation. Nature. 1988 Nov 17;336(6196):244–246. doi: 10.1038/336244a0. [DOI] [PubMed] [Google Scholar]