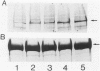
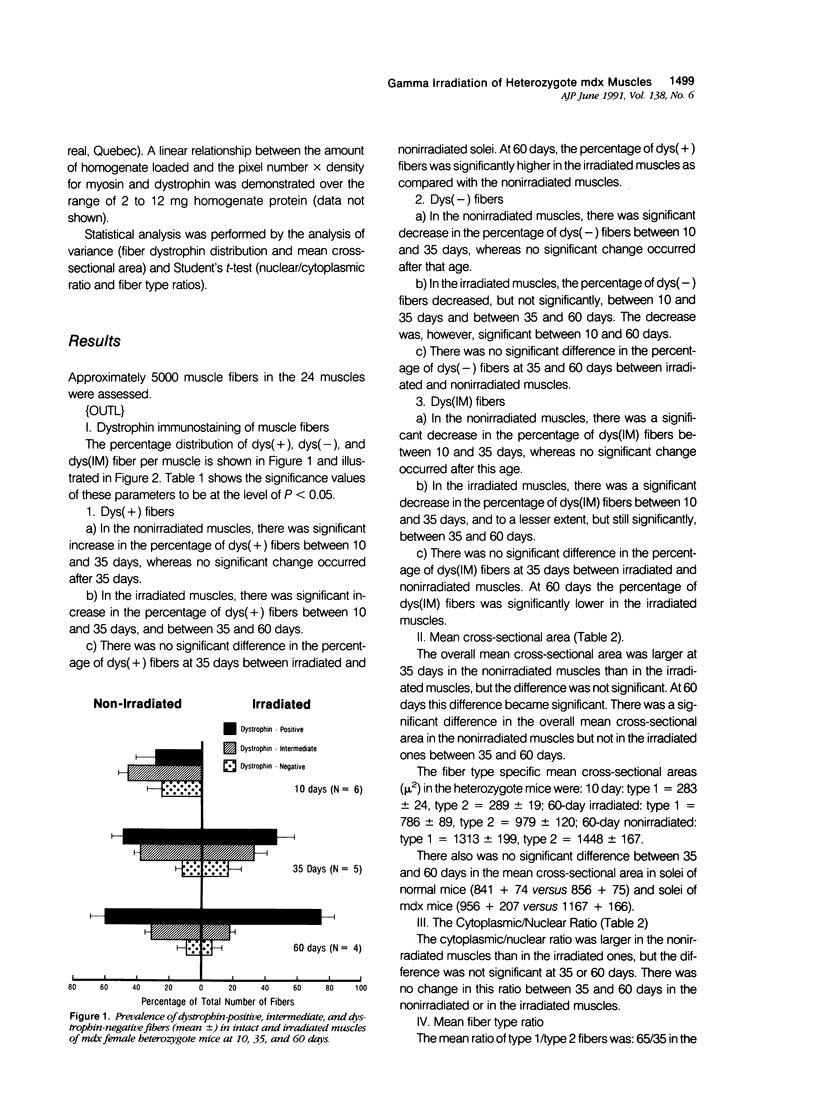
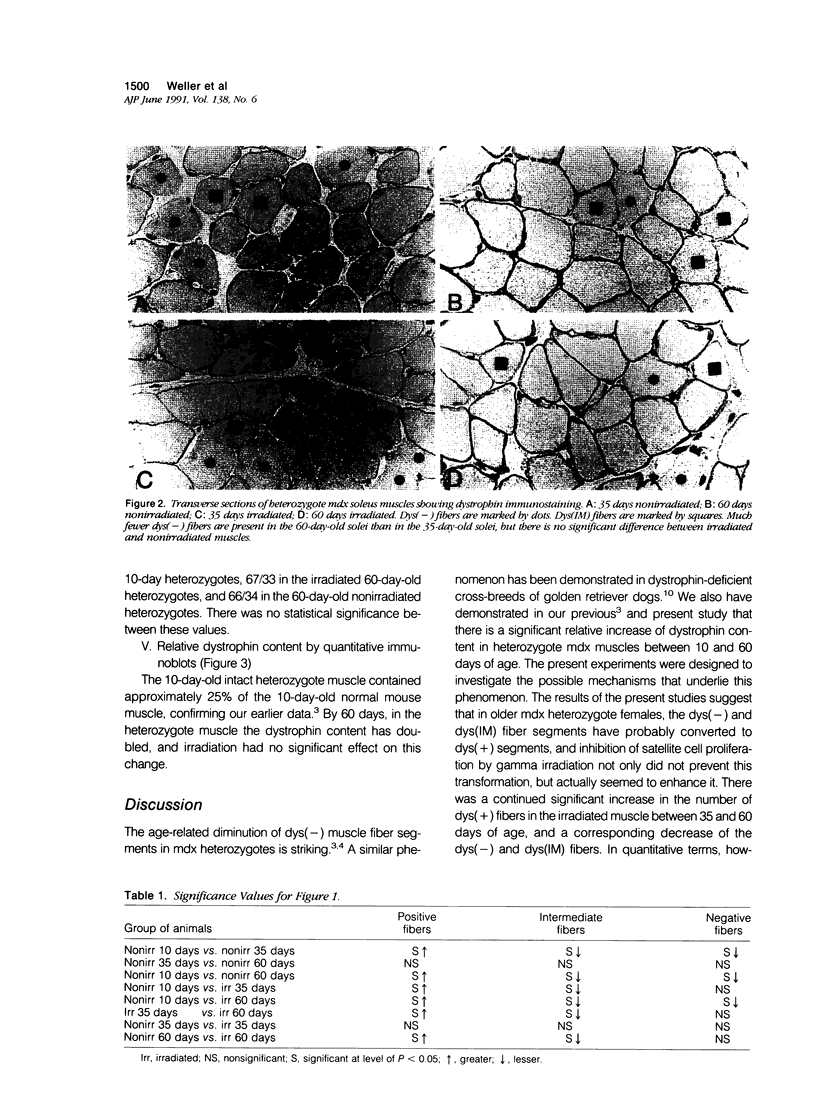
Abstract
In skeletal muscles of young mdx female heterozygote mice, there is a mosaic of dystrophin-positive and dystrophin-negative fiber segments. In older animals, there is a marked decline in the number of dystrophin-negative fiber segments. This phenomenon might be due to a fusion of dystrophin-competent satellite cells into the originally dystrophin-negative fiber segments during growth. To study this possibility, soleus muscles of 10-day-old mdx female heterozygotes were gamma irradiated (2000 rads) to inhibit subsequent myosatellite cell proliferation and fusion. In the irradiated soleus muscles of animals at 60 days, the relative amount of dystrophin measured by quantitative immunoblots was not significantly different from that of the contralateral nonirradiated muscles. The prevalence of dystrophin-negative fibers in the 60-day-old irradiated solei was not higher than in the nonirradiated contralateral muscles, implying that dystrophin-competent satellite cell fusion was not a significant factor in the observed conversion. A longitudinal expansion of the cytoplasmic domain of the original dystrophin-competent myonuclei during growth could explain the observed conversion phenomenon.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Campbell K. P., Kahl S. D. Association of dystrophin and an integral membrane glycoprotein. Nature. 1989 Mar 16;338(6212):259–262. doi: 10.1038/338259a0. [DOI] [PubMed] [Google Scholar]
- Cooper B. J., Gallagher E. A., Smith C. A., Valentine B. A., Winand N. J. Mosaic expression of dystrophin in carriers of canine X-linked muscular dystrophy. Lab Invest. 1990 Feb;62(2):171–178. [PubMed] [Google Scholar]
- Gartler S. M., Riggs A. D. Mammalian X-chromosome inactivation. Annu Rev Genet. 1983;17:155–190. doi: 10.1146/annurev.ge.17.120183.001103. [DOI] [PubMed] [Google Scholar]
- Gulati A. K. The effect of X-irradiation on skeletal muscle regeneration in the adult rat. J Neurol Sci. 1987 Mar;78(1):111–120. doi: 10.1016/0022-510x(87)90083-9. [DOI] [PubMed] [Google Scholar]
- Ishikawa H. Electron microscopic observations of satellite cells with special reference to the development of mammalian skeletal muscles. Z Anat Entwicklungsgesch. 1966;125(1):43–63. doi: 10.1007/BF00521974. [DOI] [PubMed] [Google Scholar]
- Karpati G., Carpenter S., Prescott S. Small-caliber skeletal muscle fibers do not suffer necrosis in mdx mouse dystrophy. Muscle Nerve. 1988 Aug;11(8):795–803. doi: 10.1002/mus.880110802. [DOI] [PubMed] [Google Scholar]
- Karpati G., Pouliot Y., Zubrzycka-Gaarn E., Carpenter S., Ray P. N., Worton R. G., Holland P. Dystrophin is expressed in mdx skeletal muscle fibers after normal myoblast implantation. Am J Pathol. 1989 Jul;135(1):27–32. [PMC free article] [PubMed] [Google Scholar]
- Karpati G., Zubrzycka-Gaarn E. E., Carpenter S., Bulman D. E., Ray P. N., Worton R. G. Age-related conversion of dystrophin-negative to -positive fiber segments of skeletal but not cardiac muscle fibers in heterozygote mdx mice. J Neuropathol Exp Neurol. 1990 Mar;49(2):96–105. doi: 10.1097/00005072-199003000-00002. [DOI] [PubMed] [Google Scholar]
- Kelly A. M. Satellite cells and myofiber growth in the rat soleus and extensor digitorum longus muscles. Dev Biol. 1978 Jul;65(1):1–10. doi: 10.1016/0012-1606(78)90174-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Partridge T. A., Morgan J. E., Coulton G. R., Hoffman E. P., Kunkel L. M. Conversion of mdx myofibres from dystrophin-negative to -positive by injection of normal myoblasts. Nature. 1989 Jan 12;337(6203):176–179. doi: 10.1038/337176a0. [DOI] [PubMed] [Google Scholar]
- Schultz E., Jaryszak D. L., Valliere C. R. Response of satellite cells to focal skeletal muscle injury. Muscle Nerve. 1985 Mar-Apr;8(3):217–222. doi: 10.1002/mus.880080307. [DOI] [PubMed] [Google Scholar]
- Sicinski P., Geng Y., Ryder-Cook A. S., Barnard E. A., Darlison M. G., Barnard P. J. The molecular basis of muscular dystrophy in the mdx mouse: a point mutation. Science. 1989 Jun 30;244(4912):1578–1580. doi: 10.1126/science.2662404. [DOI] [PubMed] [Google Scholar]
- Tanaka H., Ikeya K., Ozawa E. Difference in the expression pattern of dystrophin on the surface membrane between the skeletal and cardiac muscles of mdx carrier mice. Histochemistry. 1990;93(5):447–452. doi: 10.1007/BF00266399. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakeford S., Watt D. J., Partridge T. A. X-irradiation improves mdx mouse muscle as a model of myofiber loss in DMD. Muscle Nerve. 1991 Jan;14(1):42–50. doi: 10.1002/mus.880140108. [DOI] [PubMed] [Google Scholar]
- Watkins S. C., Hoffman E. P., Slayter H. S., Kunkel L. M. Dystrophin distribution in heterozygote MDX mice. Muscle Nerve. 1989 Oct;12(10):861–868. doi: 10.1002/mus.880121013. [DOI] [PubMed] [Google Scholar]