Abstract
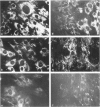
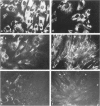
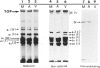
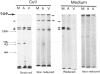
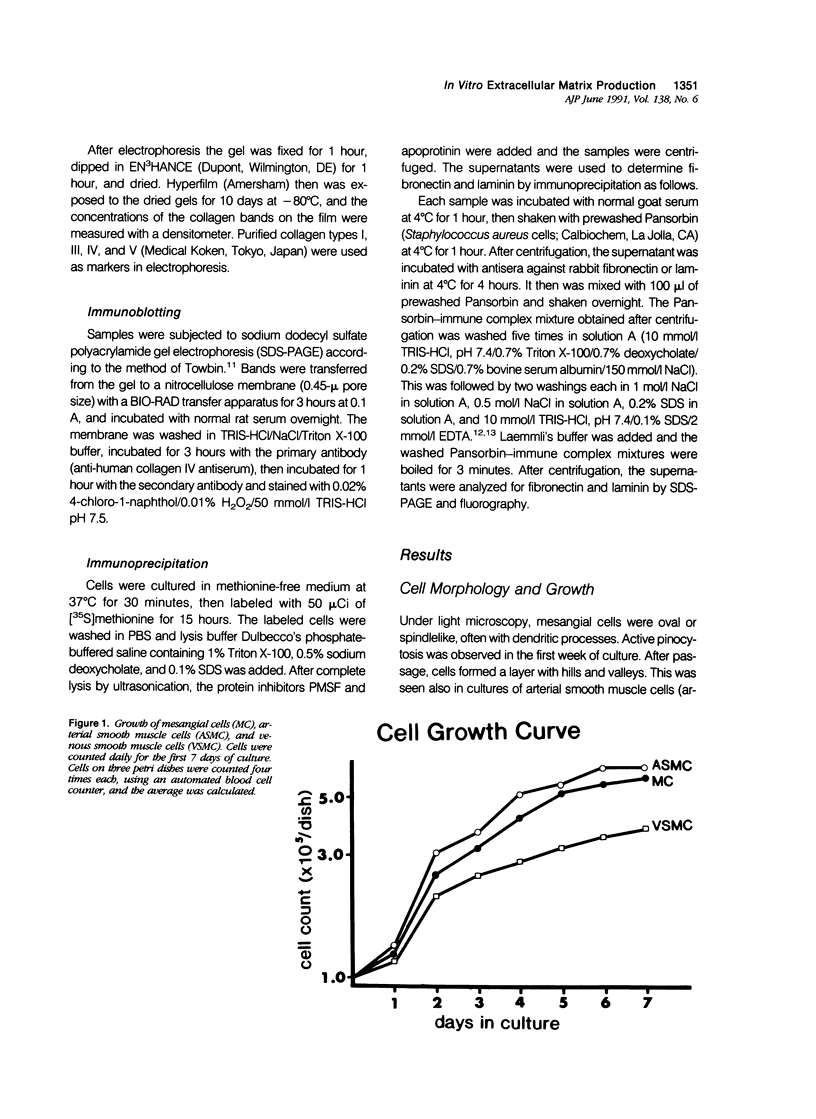
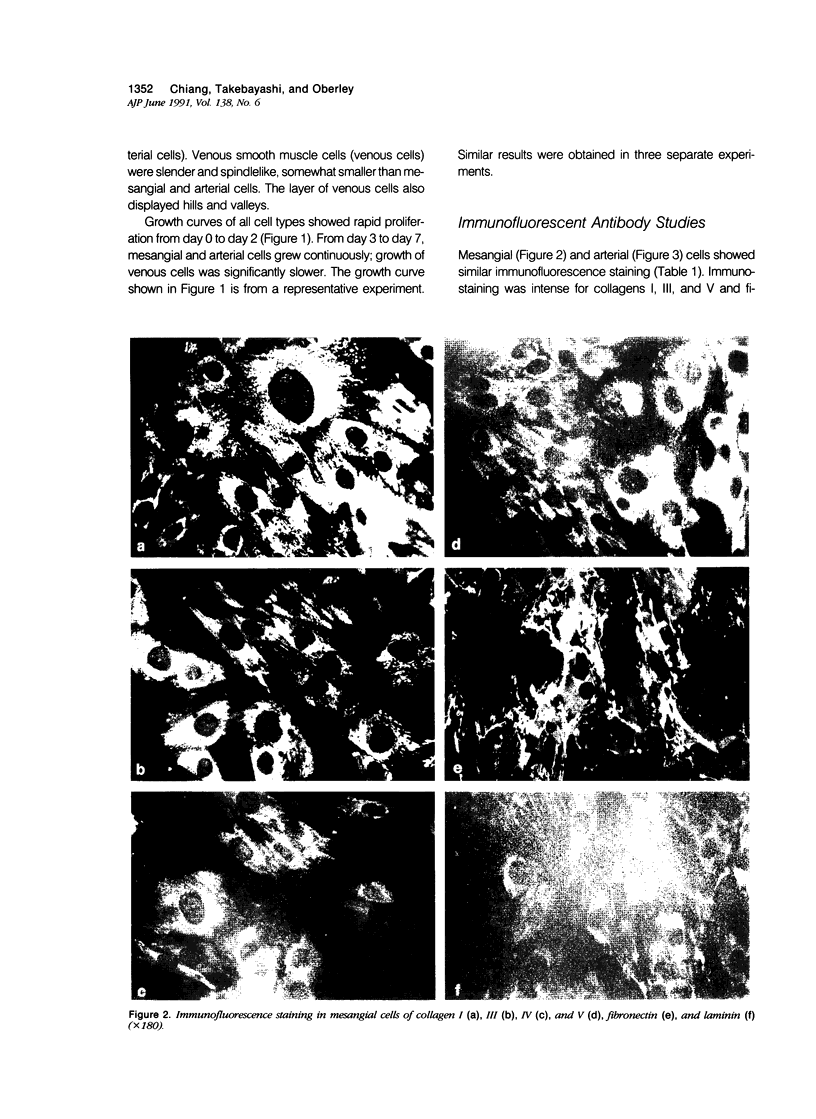
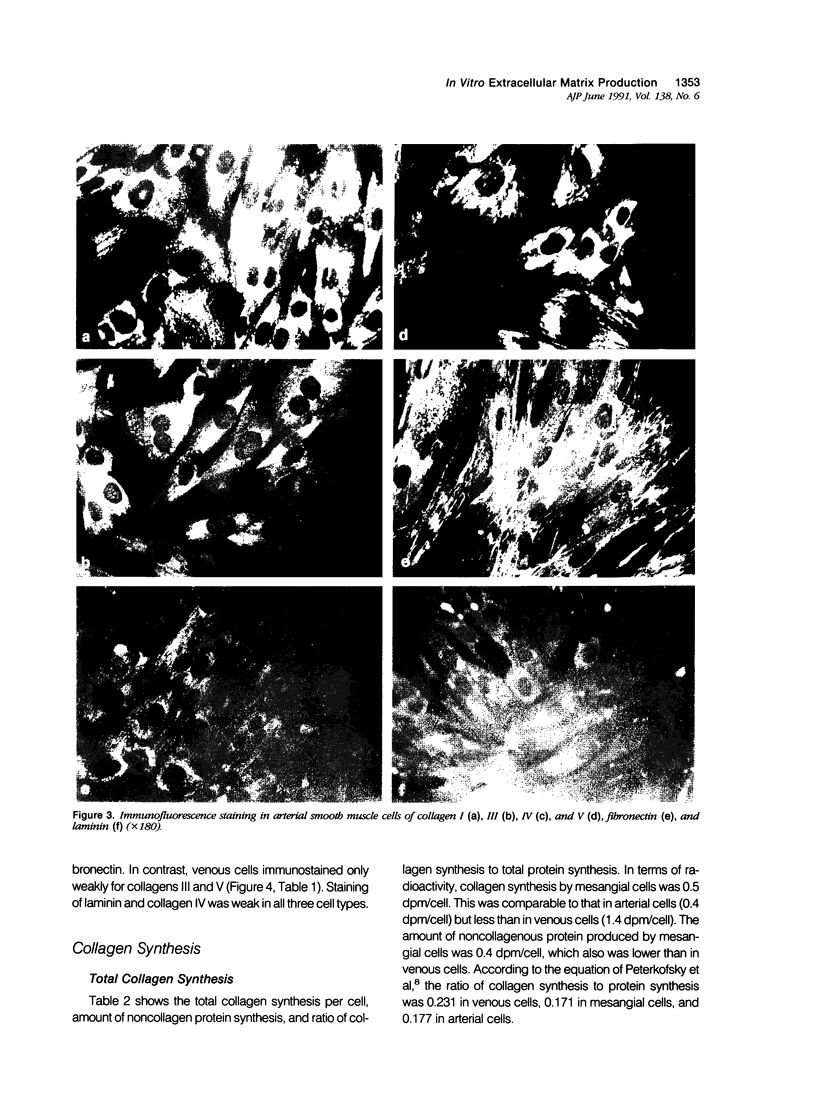
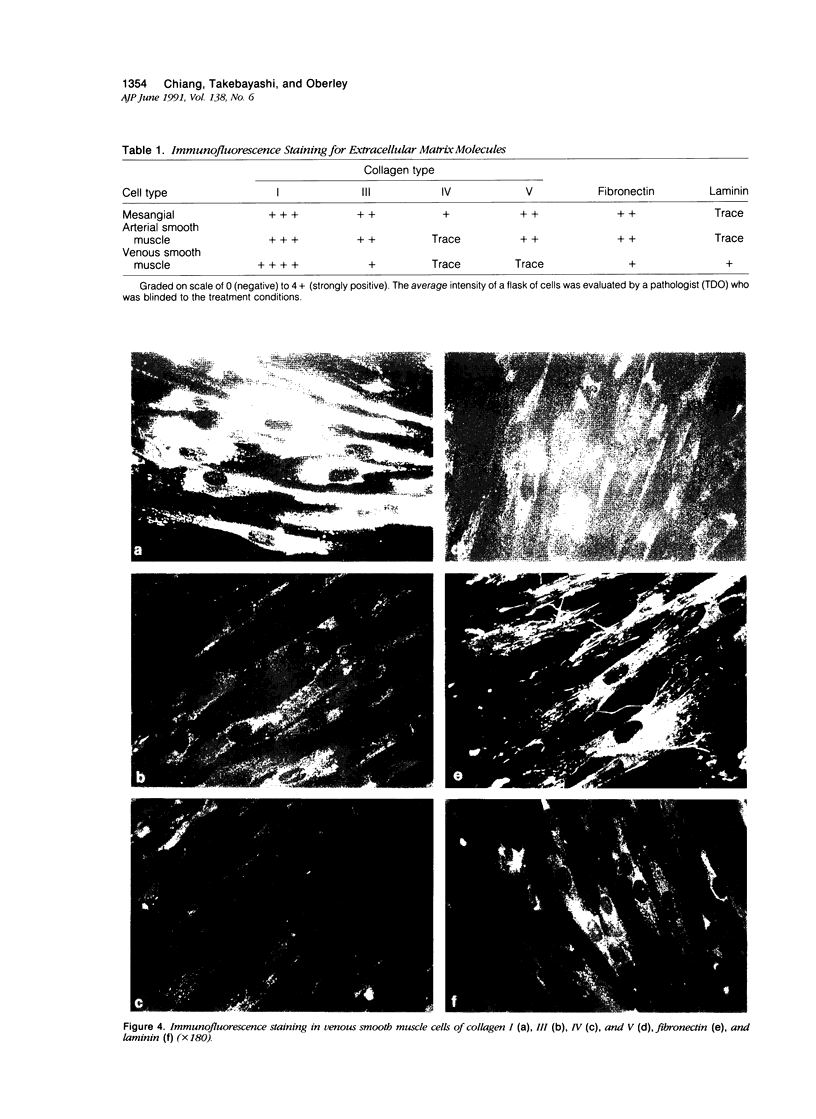
Proliferation potential and extracellular matrix production were compared in cultured porcine glomerular mesangial cells and arterial and venous smooth muscle cells. Mesangial and arterial smooth muscle cells proliferated more rapidly than venous smooth muscle cells. In immunofluorescence studies, mesangial and arterial smooth muscle cells stained strongly for collagen types I, III, and V; venous smooth muscles showed weaker staining for collagens III and V. Total collagen synthesis in cultured mesangial and arterial smooth muscle cells was lower than in venous smooth muscle cells. Electrophoretic analysis showed type I collagen predominated in all cell types, although levels were highest in mesangial and arterial smooth muscle cells. Collagen V (alpha 3) occurred only in venous smooth muscle cells. Mesangial and arterial smooth muscle cells showed cellbound fibronectin and laminin, which also were secreted into the medium. Venous smooth muscle cells secreted fibronectin, but all laminin was cell bound. The findings suggest a strong similarity between mesangial and arterial smooth muscle cells.
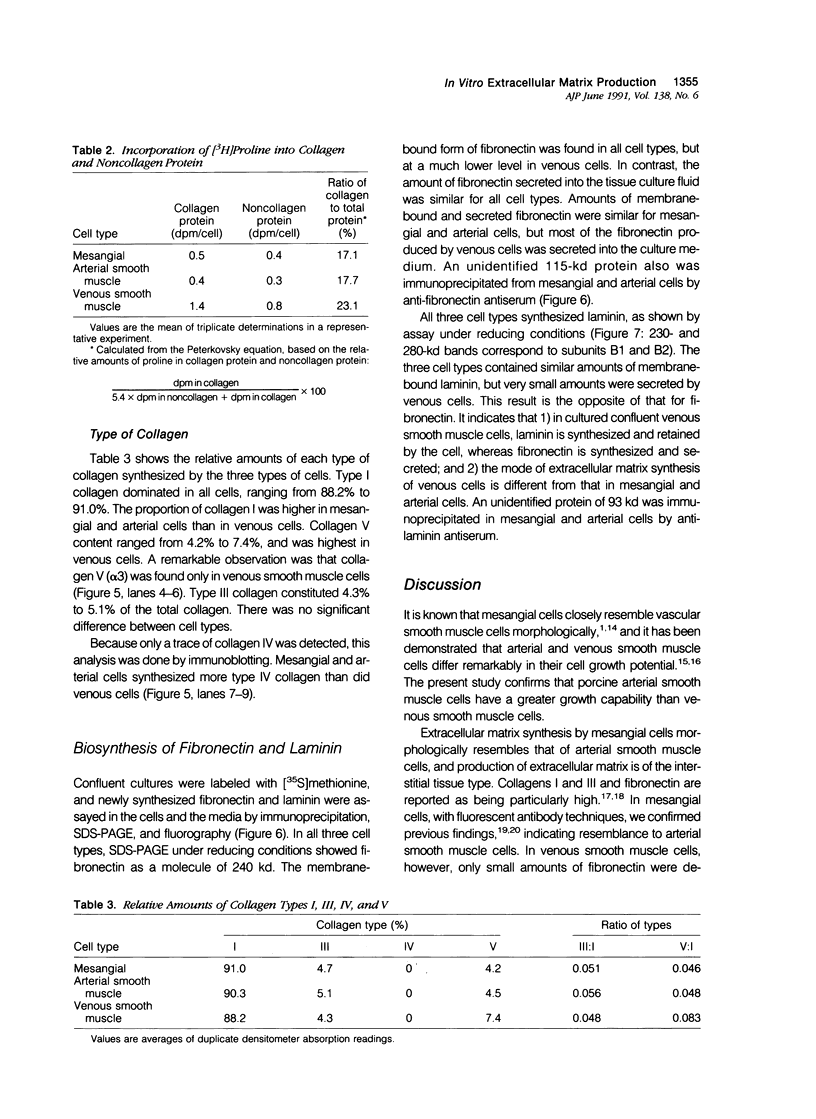
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahamson D. R., Irwin M. H., St John P. L., Perry E. W., Accavitti M. A., Heck L. W., Couchman J. R. Selective immunoreactivities of kidney basement membranes to monoclonal antibodies against laminin: localization of the end of the long arm and the short arms to discrete microdomains. J Cell Biol. 1989 Dec;109(6 Pt 2):3477–3491. doi: 10.1083/jcb.109.6.3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Van Evercooren A., Kleinman H. K., Ohno S., Marangos P., Schwartz J. P., Dubois-Dalcq M. E. Nerve growth factor, laminin, and fibronectin promote neurite growth in human fetal sensory ganglia cultures. J Neurosci Res. 1982;8(2-3):179–193. doi: 10.1002/jnr.490080208. [DOI] [PubMed] [Google Scholar]
- Couchman J. R., Hök M., Rees D. A., Timpl R. Adhesion, growth, and matrix production by fibroblasts on laminin substrates. J Cell Biol. 1983 Jan;96(1):177–183. doi: 10.1083/jcb.96.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekblom M., Klein G., Mugrauer G., Fecker L., Deutzmann R., Timpl R., Ekblom P. Transient and locally restricted expression of laminin A chain mRNA by developing epithelial cells during kidney organogenesis. Cell. 1990 Jan 26;60(2):337–346. doi: 10.1016/0092-8674(90)90748-4. [DOI] [PubMed] [Google Scholar]
- Fujikawa L. S., Foster C. S., Gipson I. K., Colvin R. B. Basement membrane components in healing rabbit corneal epithelial wounds: immunofluorescence and ultrastructural studies. J Cell Biol. 1984 Jan;98(1):128–138. doi: 10.1083/jcb.98.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D., Greenburg G., Foidart J. M., Savion N. The production and localization of laminin in cultured vascular and corneal endothelial cells. J Cell Physiol. 1981 May;107(2):171–183. doi: 10.1002/jcp.1041070203. [DOI] [PubMed] [Google Scholar]
- Haralson M. A., Jacobson H. R., Hoover R. L. Collagen polymorphism in cultured rat kidney mesangial cells. Lab Invest. 1987 Nov;57(5):513–523. [PubMed] [Google Scholar]
- Hata R., Ninomiya Y., Nagai Y., Tsukada Y. Biosynthesis of interstitial types of collagen by albumin-producing rat liver parenchymal cell (hepatocyte) clones in culture. Biochemistry. 1980 Jan 8;19(1):169–176. doi: 10.1021/bi00542a026. [DOI] [PubMed] [Google Scholar]
- Hedin U., Thyberg J. Plasma fibronectin promotes modulation of arterial smooth-muscle cells from contractile to synthetic phenotype. Differentiation. 1987;33(3):239–246. doi: 10.1111/j.1432-0436.1987.tb01563.x. [DOI] [PubMed] [Google Scholar]
- Ishimura E., Sterzel R. B., Budde K., Kashgarian M. Formation of extracellular matrix by cultured rat mesangial cells. Am J Pathol. 1989 Apr;134(4):843–855. [PMC free article] [PubMed] [Google Scholar]
- Manthorpe M., Engvall E., Ruoslahti E., Longo F. M., Davis G. E., Varon S. Laminin promotes neuritic regeneration from cultured peripheral and central neurons. J Cell Biol. 1983 Dec;97(6):1882–1890. doi: 10.1083/jcb.97.6.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberley T. D., Steinert B. W., Yang A. H., Anderson P. J. Kidney glomerular explants in serum-free media. Sequential morphologic and quantitative analysis of cell outgrowths. Virchows Arch B Cell Pathol Incl Mol Pathol. 1986;50(3):209–235. doi: 10.1007/BF02889903. [DOI] [PubMed] [Google Scholar]
- Oberley T. D., Yang A. H., Gould-Kostka J. Selection of kidney cell types in primary glomerular explant outgrowths by in vitro culture conditions. J Cell Sci. 1986 Aug;84:69–92. doi: 10.1242/jcs.84.1.69. [DOI] [PubMed] [Google Scholar]
- Perris R., Johansson S. Amphibian neural crest cell migration on purified extracellular matrix components: a chondroitin sulfate proteoglycan inhibits locomotion on fibronectin substrates. J Cell Biol. 1987 Dec;105(6 Pt 1):2511–2521. doi: 10.1083/jcb.105.6.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao C. N., Margulies I. M., Tralka T. S., Terranova V. P., Madri J. A., Liotta L. A. Isolation of a subunit of laminin and its role in molecular structure and tumor cell attachment. J Biol Chem. 1982 Aug 25;257(16):9740–9744. [PubMed] [Google Scholar]
- Rogers S. L., Letourneau P. C., Palm S. L., McCarthy J., Furcht L. T. Neurite extension by peripheral and central nervous system neurons in response to substratum-bound fibronectin and laminin. Dev Biol. 1983 Jul;98(1):212–220. doi: 10.1016/0012-1606(83)90350-0. [DOI] [PubMed] [Google Scholar]
- Ross R. The smooth muscle cell. II. Growth of smooth muscle in culture and formation of elastic fibers. J Cell Biol. 1971 Jul;50(1):172–186. doi: 10.1083/jcb.50.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinman J. I., Fish A. J., Brown D. M., Michael A. J. Human glomerular smooth muscle (mesangial) cells in culture. Lab Invest. 1976 Feb;34(2):150–158. [PubMed] [Google Scholar]
- Scheinman J. I., Foidart J. M., Michael A. F. The immunohistology of glomerular antigens. V. The collagenous antigens of the glomerulus. Lab Invest. 1980 Oct;43(4):373–381. [PubMed] [Google Scholar]
- Sterzel R. B., Lovett D. H., Foellmer H. G., Perfetto M., Biemesderfer D., Kashgarian M. Mesangial cell hillocks. Nodular foci of exaggerated growth of cells and matrix in prolonged culture. Am J Pathol. 1986 Oct;125(1):130–140. [PMC free article] [PubMed] [Google Scholar]
- Striker G. E., Striker L. J. Glomerular cell culture. Lab Invest. 1985 Aug;53(2):122–131. [PubMed] [Google Scholar]
- Sugrue S. P., Hay E. D. Response of basal epithelial cell surface and Cytoskeleton to solubilized extracellular matrix molecules. J Cell Biol. 1981 Oct;91(1):45–54. doi: 10.1083/jcb.91.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takami N., Misumi Y., Kuroki M., Matsuoka Y., Ikehara Y. Evidence for carboxyl-terminal processing and glycolipid-anchoring of human carcinoembryonic antigen. J Biol Chem. 1988 Sep 5;263(25):12716–12720. [PubMed] [Google Scholar]
- Terranova V. P., Williams J. E., Liotta L. A., Martin G. R. Modulation of the metastatic activity of melanoma cells by laminin and fibronectin. Science. 1984 Nov 23;226(4677):982–985. doi: 10.1126/science.6505678. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuru N., Jimi S., Takebayashi S. [A study on function and morphology of a mesangial cell as a arterial smooth muscle cell]. Nihon Jinzo Gakkai Shi. 1987 Jun;29(6):615–622. [PubMed] [Google Scholar]
- Vlodavsky I., Gospodarowicz D. Respective roles of laminin and fibronectin in adhesion of human carcinoma and sarcoma cells. Nature. 1981 Jan 22;289(5795):304–306. doi: 10.1038/289304a0. [DOI] [PubMed] [Google Scholar]
- Voss B., Rauterberg J. Localization of collagen types I, III, IV and V, fibronectin and laminin in human arteries by the indirect immunofluorescence method. Pathol Res Pract. 1986 Oct;181(5):568–575. doi: 10.1016/S0344-0338(86)80151-0. [DOI] [PubMed] [Google Scholar]