Abstract
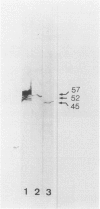
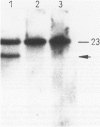
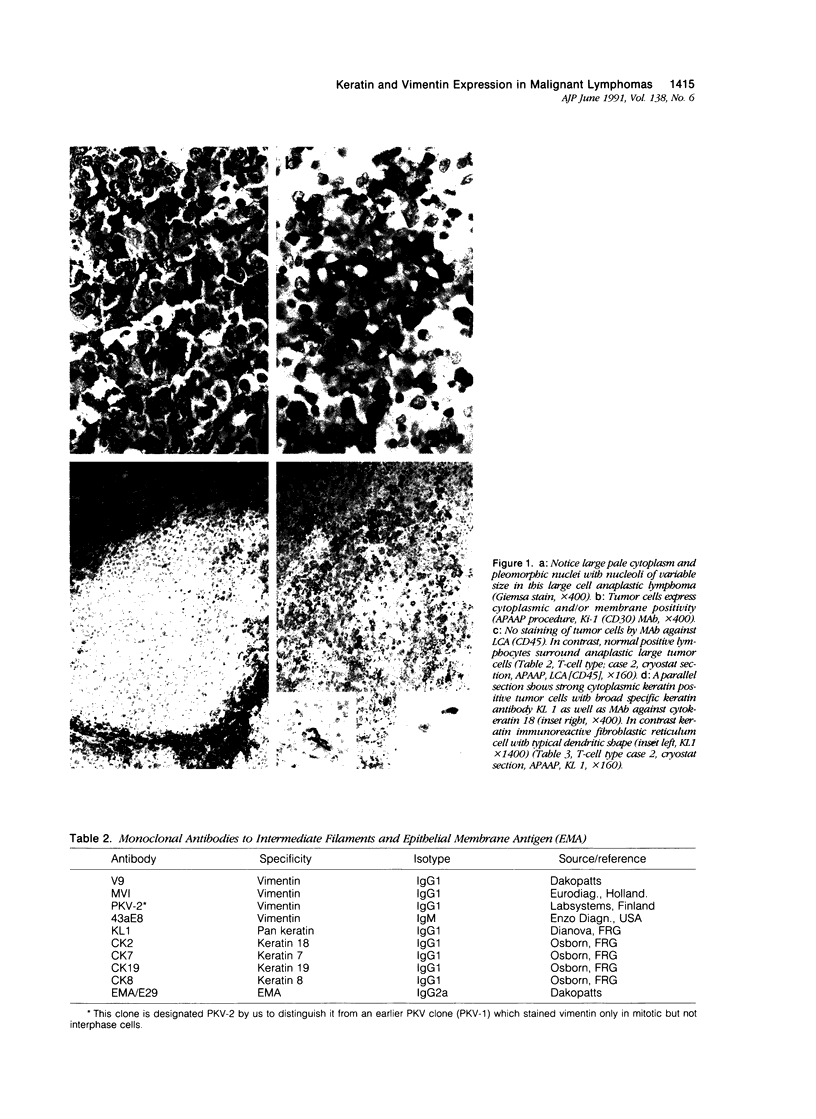
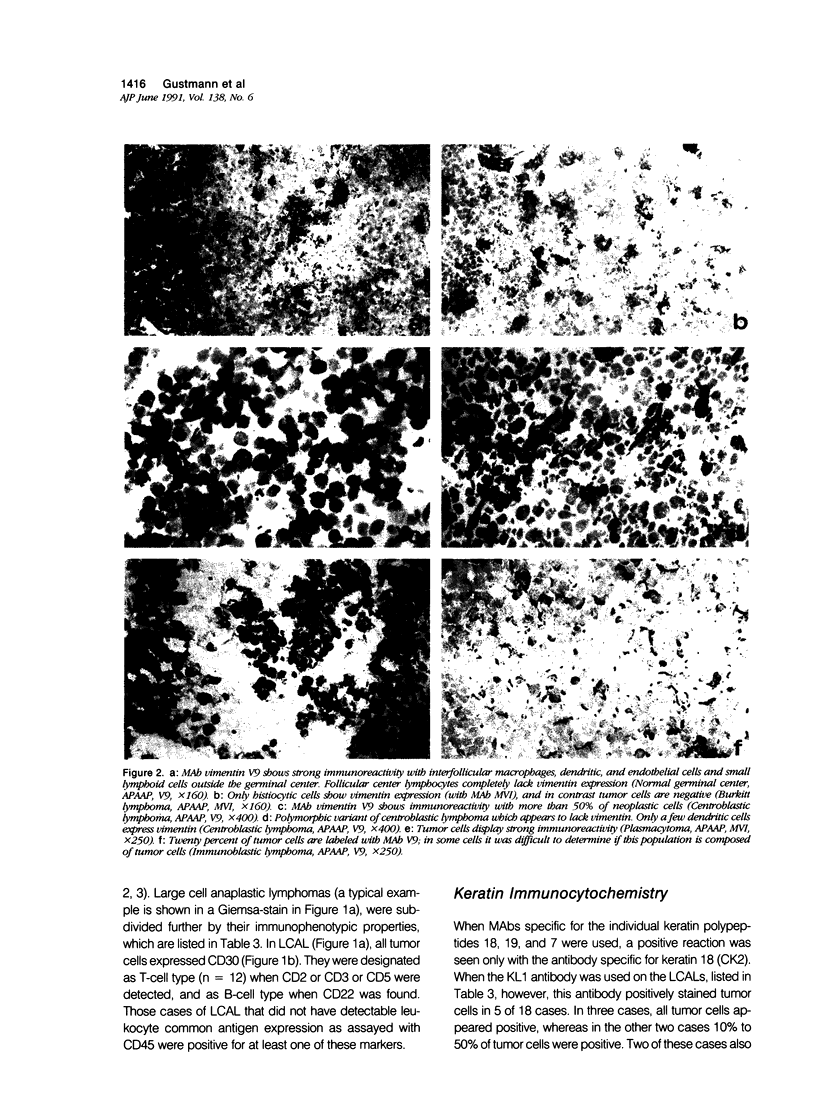
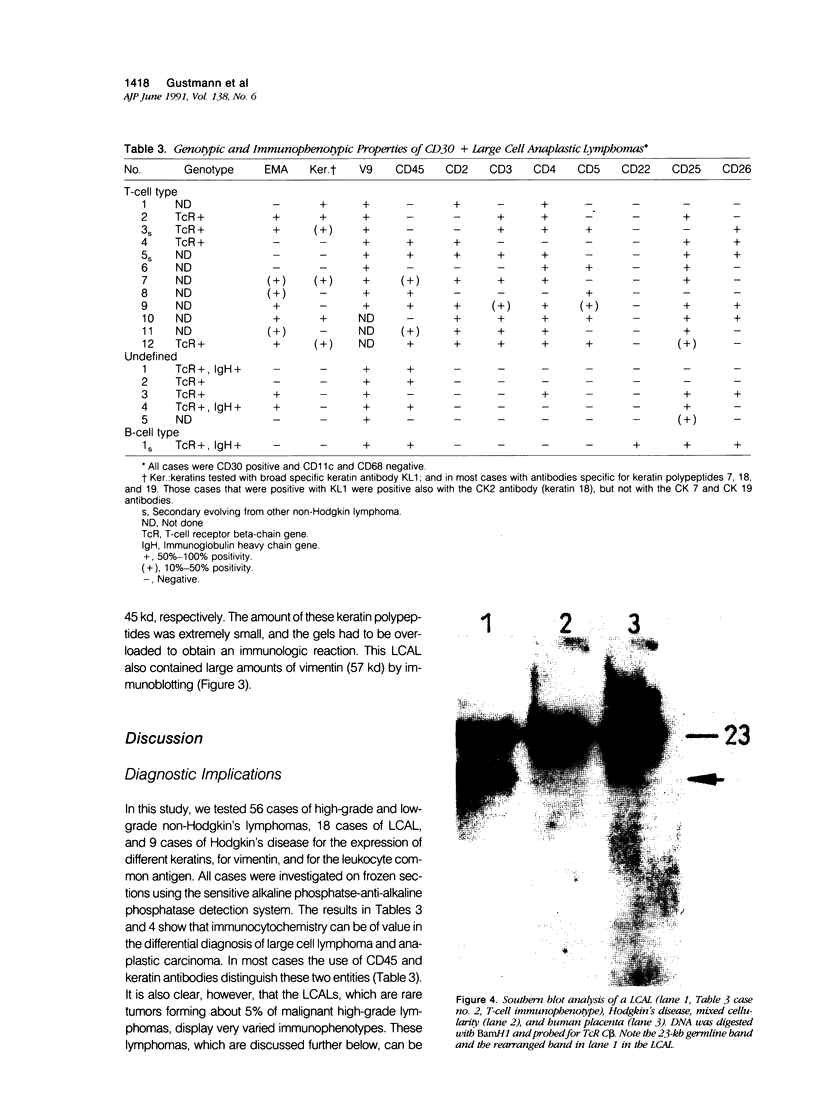
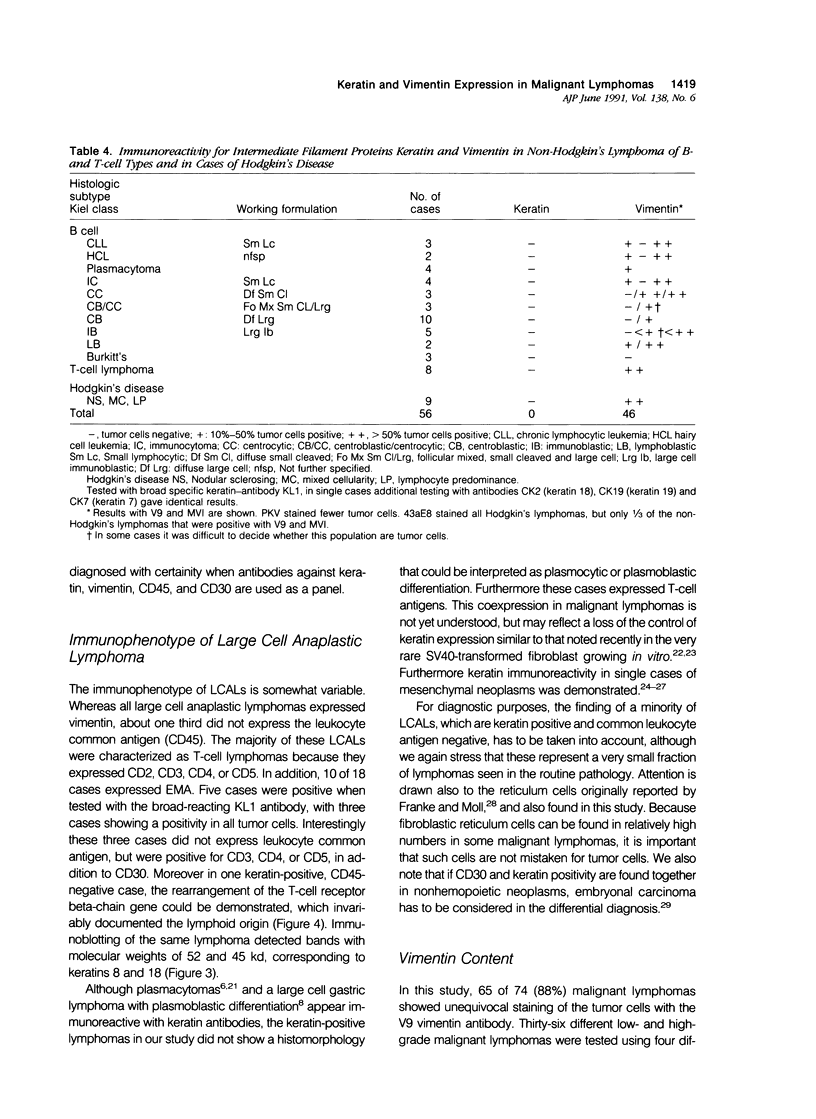
The immunophenotypes of 74 malignant lymphomas (9 Hodgkin's disease, 19 low-grade B-cell, 20 high-grade B-cell, 8 T-cell, and 18 large cell anaplastic lymphomas [LCAL]) have been characterized with antibodies against leucocyte differentiation antigens, keratin, and vimentin. All the non-LCAL were CD45 positive and keratin negative. The LCALs had a more varied immunophenotype, with CD45 present only in 11 of 18 cases and keratin present in 5 of 18 of these rare lymphomas. The lymphoid origin of these latter cases was proven by gene rearrangement studies. All LCALs were CD30+, and, where tested, vimentin positive. Of four different vimentin monoclonal antibodies tested, V9 and MVI stained the highest number of lymphomas. Positive staining of tumor cells was seen in 61 of 71 cases. Vimentin-negative cases included Burkitt's as well as some follicular lymphomas.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Coindre J. M., de Mascarel A., Trojani M., de Mascarel I., Pages A. Immunohistochemical study of rhabdomyosarcoma. Unexpected staining with S100 protein and cytokeratin. J Pathol. 1988 Jun;155(2):127–132. doi: 10.1002/path.1711550209. [DOI] [PubMed] [Google Scholar]
- Cordell J. L., Falini B., Erber W. N., Ghosh A. K., Abdulaziz Z., MacDonald S., Pulford K. A., Stein H., Mason D. Y. Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes). J Histochem Cytochem. 1984 Feb;32(2):219–229. doi: 10.1177/32.2.6198355. [DOI] [PubMed] [Google Scholar]
- Dellagi K., Brouet J. C., Portier M. M., Lenoir G. M. Abnormal expression of vimentin intermediate filaments in human lymphoid cell lines with deletion or translocation of the distal end of chromosome 8. J Natl Cancer Inst. 1984 Jul;73(1):95–100. [PubMed] [Google Scholar]
- Delsol G., Al Saati T., Gatter K. C., Gerdes J., Schwarting R., Caveriviere P., Rigal-Huguet F., Robert A., Stein H., Mason D. Y. Coexpression of epithelial membrane antigen (EMA), Ki-1, and interleukin-2 receptor by anaplastic large cell lymphomas. Diagnostic value in so-called malignant histiocytosis. Am J Pathol. 1988 Jan;130(1):59–70. [PMC free article] [PubMed] [Google Scholar]
- Domagala W., Lasota J., Chosia M., Weber K., Osborn M. Leukocyte-common antigen and vimentin are reliable adjuncts in the diagnosis of non-Hodgkin's lymphoma in fine needle aspirates. Anal Quant Cytol Histol. 1989 Feb;11(1):15–21. [PubMed] [Google Scholar]
- Dörken B., Moldenhauer G., Pezzutto A., Schwartz R., Feller A., Kiesel S., Nadler L. M. HD39 (B3), a B lineage-restricted antigen whose cell surface expression is limited to resting and activated human B lymphocytes. J Immunol. 1986 Jun 15;136(12):4470–4479. [PubMed] [Google Scholar]
- Falini B., Pileri S., Stein H., Dieneman D., Dallenbach F., Delsol G., Minelli O., Poggi S., Martelli M. F., Pallesen G. Variable expression of leucocyte-common (CD45) antigen in CD30 (Ki1)-positive anaplastic large-cell lymphoma: implications for the differential diagnosis between lymphoid and nonlymphoid malignancies. Hum Pathol. 1990 Jun;21(6):624–629. doi: 10.1016/s0046-8177(96)90009-x. [DOI] [PubMed] [Google Scholar]
- Feller A. C., Parwaresch M. R., Lennert K. Cytochemical distribution of dipeptidylaminopeptidase IV (DAP IV; EC-3.4.14.5) in T-lymphoblastic lymphoma/leukemia characterized with monoclonal antibodies. Leuk Res. 1984;8(3):397–406. doi: 10.1016/0145-2126(84)90079-1. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Grund C., Kuhn C., Lehto V. P., Virtanen I. Transient change of organization of vimentin filaments during mitosis as demonstrated by a monoclonal antibody. Exp Cell Res. 1984 Oct;154(2):567–580. doi: 10.1016/0014-4827(84)90181-2. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Moll R. Cytoskeletal components of lymphoid organs. I. Synthesis of cytokeratins 8 and 18 and desmin in subpopulations of extrafollicular reticulum cells of human lymph nodes, tonsils, and spleen. Differentiation. 1987;36(2):145–163. doi: 10.1111/j.1432-0436.1987.tb00189.x. [DOI] [PubMed] [Google Scholar]
- Gabbiani G., Kapanci Y., Barazzone P., Franke W. W. Immunochemical identification of intermediate-sized filaments in human neoplastic cells. A diagnostic aid for the surgical pathologist. Am J Pathol. 1981 Sep;104(3):206–216. [PMC free article] [PubMed] [Google Scholar]
- Gatter K. C., Abdulaziz Z., Beverley P., Corvalan J. R., Ford C., Lane E. B., Mota M., Nash J. R., Pulford K., Stein H. Use of monoclonal antibodies for the histopathological diagnosis of human malignancy. J Clin Pathol. 1982 Nov;35(11):1253–1267. doi: 10.1136/jcp.35.11.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatter K. C., Alcock C., Heryet A., Mason D. Y. Clinical importance of analysing malignant tumours of uncertain origin with immunohistological techniques. Lancet. 1985 Jun 8;1(8441):1302–1305. doi: 10.1016/s0140-6736(85)92794-1. [DOI] [PubMed] [Google Scholar]
- Giorno R., Sciotto C. G. Use of monoclonal antibodies for analyzing the distribution of the intermediate filament protein vimentin in human non-Hodgkin's lymphomas. Am J Pathol. 1985 Sep;120(3):351–355. [PMC free article] [PubMed] [Google Scholar]
- Gown A. M., Vogel A. M. Monoclonal antibodies to human intermediate filament proteins. III. Analysis of tumors. Am J Clin Pathol. 1985 Oct;84(4):413–424. doi: 10.1093/ajcp/84.4.413. [DOI] [PubMed] [Google Scholar]
- Hirose T., Kudo E., Hasegawa T., Abe J., Hizawa K. Expression of intermediate filaments in malignant fibrous histiocytomas. Hum Pathol. 1989 Sep;20(9):871–877. doi: 10.1016/0046-8177(89)90099-3. [DOI] [PubMed] [Google Scholar]
- Knapp A. C., Franke W. W. Spontaneous losses of control of cytokeratin gene expression in transformed, non-epithelial human cells occurring at different levels of regulation. Cell. 1989 Oct 6;59(1):67–79. doi: 10.1016/0092-8674(89)90870-2. [DOI] [PubMed] [Google Scholar]
- Lilienbaum A., Legagneux V., Portier M. M., Dellagi K., Paulin D. Vimentin gene: expression in human lymphocytes and in Burkitt's lymphoma cells. EMBO J. 1986 Nov;5(11):2809–2814. doi: 10.1002/j.1460-2075.1986.tb04572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miettinen M. Immunoreactivity for cytokeratin and epithelial membrane antigen in leiomyosarcoma. Arch Pathol Lab Med. 1988 Jun;112(6):637–640. [PubMed] [Google Scholar]
- Möller P., Momburg F., Hofmann W. J., Matthaei-Maurer D. U. Lack of vimentin occurring during the intrafollicular stages of B cell development characterizes follicular center cell lymphomas. Blood. 1988 Apr;71(4):1033–1038. [PubMed] [Google Scholar]
- Nagle R. B. Intermediate filaments: a review of the basic biology. Am J Surg Pathol. 1988;12 (Suppl 1):4–16. [PubMed] [Google Scholar]
- Osborn M., Weber K. Tumor diagnosis by intermediate filament typing: a novel tool for surgical pathology. Lab Invest. 1983 Apr;48(4):372–394. [PubMed] [Google Scholar]
- Pallesen G., Hamilton-Dutoit S. J. Ki-1 (CD30) antigen is regularly expressed by tumor cells of embryonal carcinoma. Am J Pathol. 1988 Dec;133(3):446–450. [PMC free article] [PubMed] [Google Scholar]
- Pfreundschuh M., Mommertz E., Meissner M., Feller A. C., Hassa R., Krueger G. R., Diehl V. Hodgkin and Reed-Sternberg cell associated monoclonal antibodies HRS-1 and HRS-2 react with activated cells of lymphoid and monocytoid origin. Anticancer Res. 1988 Mar-Apr;8(2):217–224. [PubMed] [Google Scholar]
- Pizzolo G., Sloane J., Beverley P., Thomas J. A., Bradstock K. F., Mattingly S., Janossy G. Differential diagnosis of malignant lymphoma and nonlymphoid tumors using monoclonal anti-leucocyte antibody. Cancer. 1980 Dec 15;46(12):2640–2647. doi: 10.1002/1097-0142(19801215)46:12<2640::aid-cncr2820461218>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Radzun H. J., Parwaresch M. R. Differential immunohistochemical resolution of the human mononuclear phagocyte system. Cell Immunol. 1983 Nov;82(1):174–183. doi: 10.1016/0008-8749(83)90151-x. [DOI] [PubMed] [Google Scholar]
- Ramaekers F. C., Puts J. J., Kant A., Moesker O., Jap P. H., Vooijs G. P. Use of antibodies to intermediate filaments in the characterization of human tumors. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 1):331–339. doi: 10.1101/sqb.1982.046.01.034. [DOI] [PubMed] [Google Scholar]
- Ramaekers F. C., Vroom T. M., Moesker O., Kant A., Scholte G., Vooijs G. P. The use of antibodies to intermediate filament proteins in the differential diagnosis of lymphoma versus metastatic carcinoma. Histochem J. 1985 Jan;17(1):57–70. doi: 10.1007/BF01003403. [DOI] [PubMed] [Google Scholar]
- Ravetch J. V., Siebenlist U., Korsmeyer S., Waldmann T., Leder P. Structure of the human immunoglobulin mu locus: characterization of embryonic and rearranged J and D genes. Cell. 1981 Dec;27(3 Pt 2):583–591. doi: 10.1016/0092-8674(81)90400-1. [DOI] [PubMed] [Google Scholar]
- Sewell H. F., Thompson W. D., King D. J. IgD myeloma/immunoblastic lymphoma cells expressing cytokeratin. Br J Cancer. 1986 May;53(5):695–696. doi: 10.1038/bjc.1986.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stansfeld A. G., Diebold J., Noel H., Kapanci Y., Rilke F., Kelényi G., Sundstrom C., Lennert K., van Unnik J. A., Mioduszewska O. Updated Kiel classification for lymphomas. Lancet. 1988 Feb 6;1(8580):292–293. doi: 10.1016/s0140-6736(88)90367-4. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama T., Broder S., Waldmann T. A. A monoclonal antibody (anti-Tac) reactive with activated and functionally mature human T cells. I. Production of anti-Tac monoclonal antibody and distribution of Tac (+) cells. J Immunol. 1981 Apr;126(4):1393–1397. [PubMed] [Google Scholar]
- Virtanen I., Miettinen M., Lehto V. P., Kariniemi A. L., Paasivuo R. Diagnostic application of monoclonal antibodies to intermediate filaments. Ann N Y Acad Sci. 1985;455:635–648. doi: 10.1111/j.1749-6632.1985.tb50441.x. [DOI] [PubMed] [Google Scholar]
- Virtanen I., Närvänen O., Lehto V. P. Differential immunoreactivity and Ca2+-dependent degradation of vimentin in human fibroblasts and fibrosarcoma cells. Int J Cancer. 1988 Aug 15;42(2):256–260. doi: 10.1002/ijc.2910420219. [DOI] [PubMed] [Google Scholar]
- Warnke R. A., Gatter K. C., Falini B., Hildreth P., Woolston R. E., Pulford K., Cordell J. L., Cohen B., De Wolf-Peeters C., Mason D. Y. Diagnosis of human lymphoma with monoclonal antileukocyte antibodies. N Engl J Med. 1983 Nov 24;309(21):1275–1281. doi: 10.1056/NEJM198311243092102. [DOI] [PubMed] [Google Scholar]
- Wotherspoon A. C., Norton A. J., Isaacson P. G. Immunoreactive cytokeratins in plasmacytomas. Histopathology. 1989 Feb;14(2):141–150. doi: 10.1111/j.1365-2559.1989.tb02124.x. [DOI] [PubMed] [Google Scholar]
- Yoshikai Y., Anatoniou D., Clark S. P., Yanagi Y., Sangster R., Van den Elsen P., Terhorst C., Mak T. W. Sequence and expression of transcripts of the human T-cell receptor beta-chain genes. Nature. 1984 Dec 6;312(5994):521–524. doi: 10.1038/312521a0. [DOI] [PubMed] [Google Scholar]
- de Mascarel A., Merlio J. P., Coindre J. M., Goussot J. F., Broustet A. Gastric large cell lymphoma expressing cytokeratin but no leukocyte common antigen. A diagnostic dilemma. Am J Clin Pathol. 1989 Apr;91(4):478–481. doi: 10.1093/ajcp/91.4.478. [DOI] [PubMed] [Google Scholar]