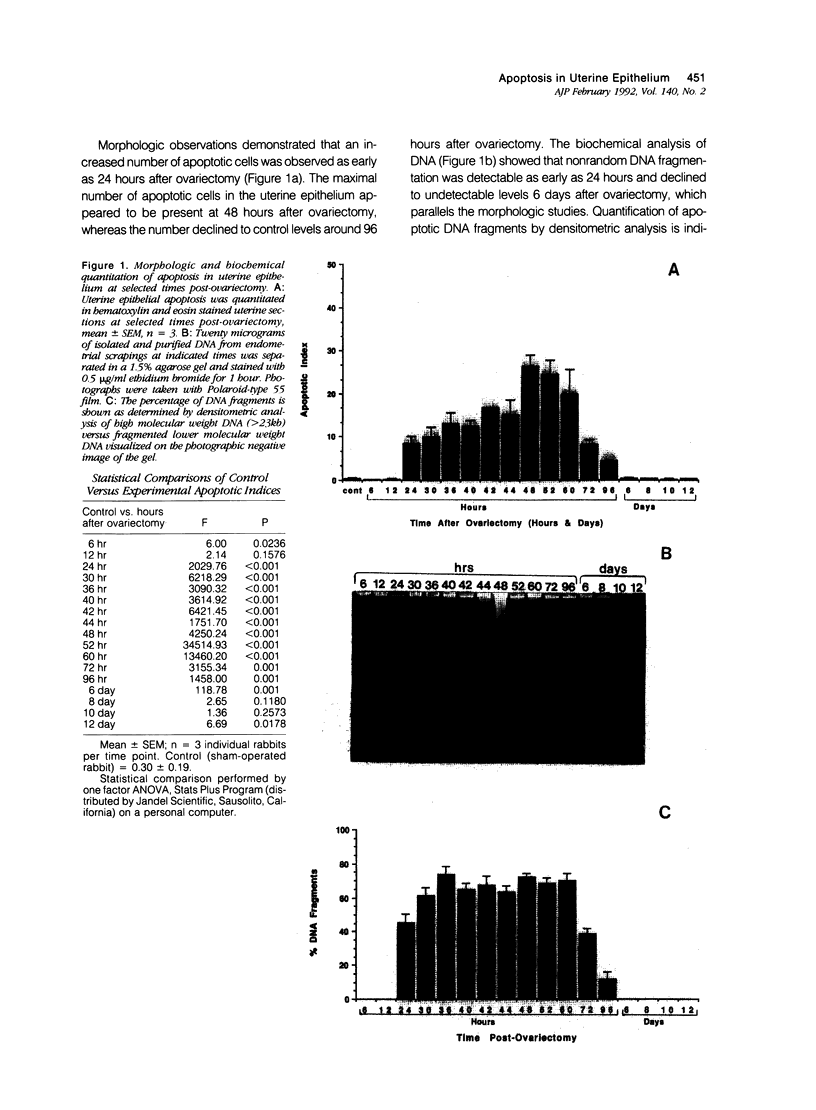
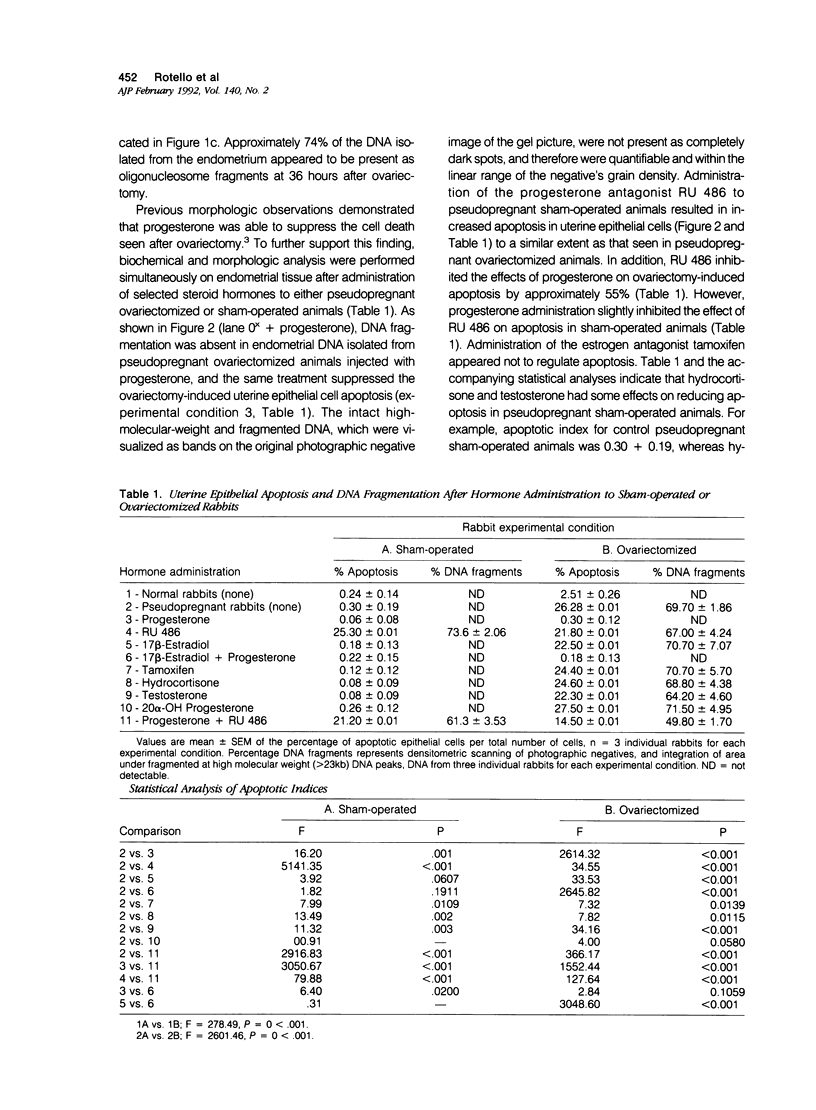
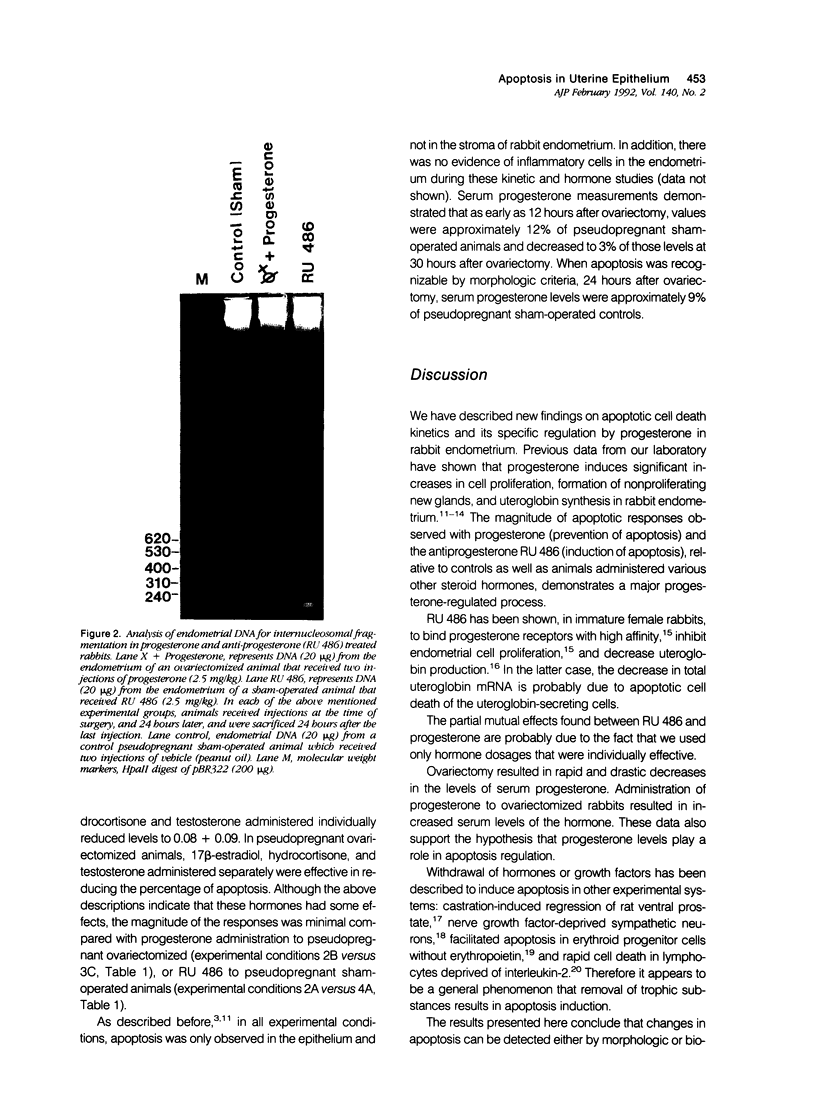
Abstract
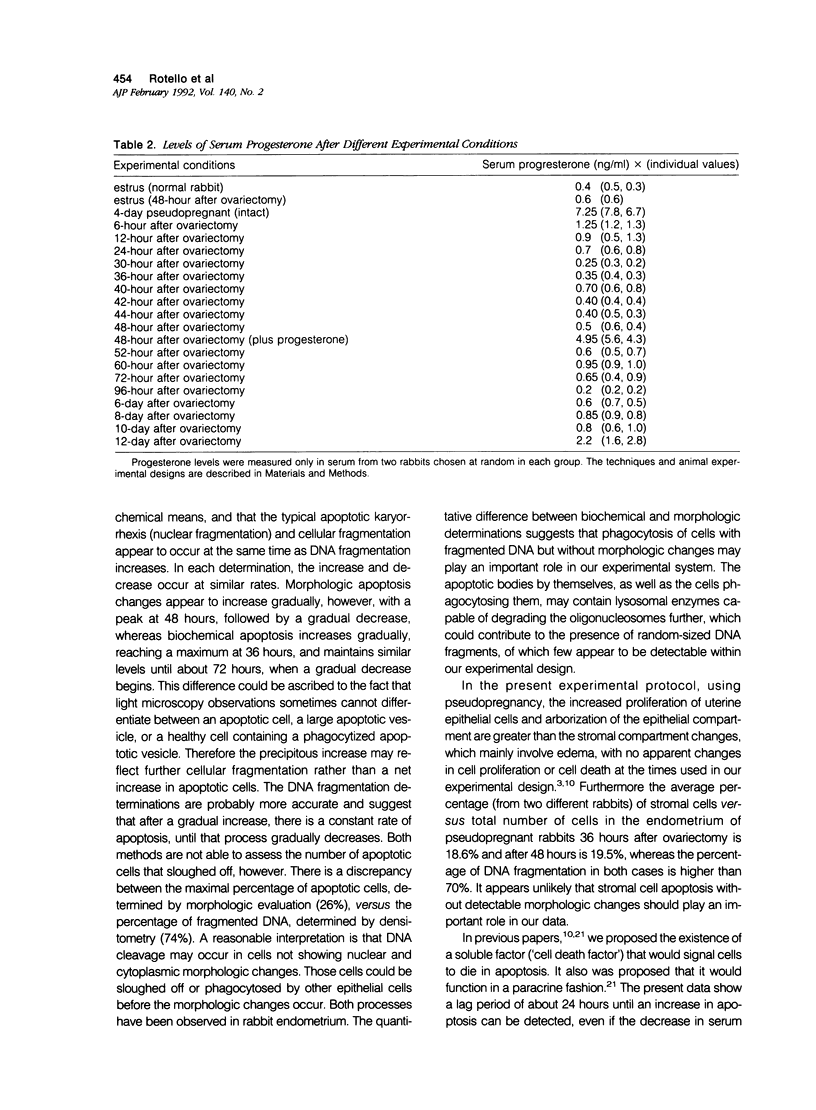
The authors show here that progesterone suppresses apoptosis, and its antagonist RU 486 induces it in rabbit uterine epithelium, as assessed by morphologic and biochemical studies. The authors' studies demonstrate that internucleosomal DNA fragments are identifiable as early as 24 hours after ovariectomy of pseudopregnant rabbits, and become undetectable 6 days after ovariectomy. Maximal levels of DNA fragmentation (about 74% of total isolated DNA) were observed 36 hours after ovariectomy. The number of apoptotic cells appeared to increase parallel to the increased DNA breakdown, and accounted for approximately 26% of the uterine epithelial cells at 48 hours after ovariectomy. Levels of progesterone in serum dropped precipitously 6 hours after ovariectomy and remained very low for several days. Administration of progesterone, more than any other steroid hormone, to pseudopregnant ovariectomized rabbits, prevented the increase in apoptotic cell death. By contrast, administration of the anti-progestin RU 486 to pseudopregnant rabbits triggered apoptosis, which attained levels similar to those observed in ovariectomized animals. The authors' findings establish that uterine epithelium apoptosis occurs in a time-dependent fashion and provides strong evidence that the actions of progesterone in that tissue are not only to stimulate cell proliferation and differentiation, but also to suppress apoptosis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buttyan R., Zakeri Z., Lockshin R., Wolgemuth D. Cascade induction of c-fos, c-myc, and heat shock 70K transcripts during regression of the rat ventral prostate gland. Mol Endocrinol. 1988 Jul;2(7):650–657. doi: 10.1210/mend-2-7-650. [DOI] [PubMed] [Google Scholar]
- Conti C. J., Gimenez-Conti I. B., Zerbe G. O., Gerschenson L. E. Differential effects of estradiol-17 beta and progesterone on the proliferation of glandular and luminal cells of rabbit uterine epithelium. Biol Reprod. 1981 Apr;24(3):643–648. doi: 10.1095/biolreprod24.3.643. [DOI] [PubMed] [Google Scholar]
- Conti C. J., Gimenez-Conti I. B., Zerbe G. O., Gerschenson L. E. Differential effects of estradiol-17 beta and progesterone on the proliferation of glandular and luminal cells of rabbit uterine epithelium. Biol Reprod. 1981 Apr;24(3):643–648. doi: 10.1095/biolreprod24.3.643. [DOI] [PubMed] [Google Scholar]
- Duke R. C., Cohen J. J. IL-2 addiction: withdrawal of growth factor activates a suicide program in dependent T cells. Lymphokine Res. 1986 Fall;5(4):289–299. [PubMed] [Google Scholar]
- Ellis H. M., Horvitz H. R. Genetic control of programmed cell death in the nematode C. elegans. Cell. 1986 Mar 28;44(6):817–829. doi: 10.1016/0092-8674(86)90004-8. [DOI] [PubMed] [Google Scholar]
- Kerr J. F., Searle J. Deletion of cells by apoptosis during castration-induced involution of the rat prostate. Virchows Arch B Cell Pathol. 1973 Jun 25;13(2):87–102. doi: 10.1007/BF02889300. [DOI] [PubMed] [Google Scholar]
- Koury M. J., Bondurant M. C. Erythropoietin retards DNA breakdown and prevents programmed death in erythroid progenitor cells. Science. 1990 Apr 20;248(4953):378–381. doi: 10.1126/science.2326648. [DOI] [PubMed] [Google Scholar]
- Kyprianou N., Isaacs J. T. Activation of programmed cell death in the rat ventral prostate after castration. Endocrinology. 1988 Feb;122(2):552–562. doi: 10.1210/endo-122-2-552. [DOI] [PubMed] [Google Scholar]
- Kyprianou N., Isaacs J. T. Expression of transforming growth factor-beta in the rat ventral prostate during castration-induced programmed cell death. Mol Endocrinol. 1989 Oct;3(10):1515–1522. doi: 10.1210/mend-3-10-1515. [DOI] [PubMed] [Google Scholar]
- Lynch M. P., Nawaz S., Gerschenson L. E. Evidence for soluble factors regulating cell death and cell proliferation in primary cultures of rabbit endometrial cells grown on collagen. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4784–4788. doi: 10.1073/pnas.83.13.4784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D. P., Schmidt R. E., DiStefano P. S., Lowry O. H., Carter J. G., Johnson E. M., Jr Inhibitors of protein synthesis and RNA synthesis prevent neuronal death caused by nerve growth factor deprivation. J Cell Biol. 1988 Mar;106(3):829–844. doi: 10.1083/jcb.106.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawaz S., Lynch M. P., Galand P., Gerschenson L. E. Hormonal regulation of cell death in rabbit uterine epithelium. Am J Pathol. 1987 Apr;127(1):51–59. [PMC free article] [PubMed] [Google Scholar]
- Rajkumar K., Bigsby R., Lieberman R., Gerschenson L. E. Effect of progesterone and 17 beta-estradiol on the production of uteroglobin by cultured rabbit uterine epithelial cells. Endocrinology. 1983 Apr;112(4):1499–1505. doi: 10.1210/endo-112-4-1499. [DOI] [PubMed] [Google Scholar]
- Rauch M., Loosfelt H., Philibert D., Milgrom E. Mechanism of action of an antiprogesterone, RU486, in the rabbit endometrium. Effects of RU486 on the progesterone receptor and on the expression of the uteroglobin gene. Eur J Biochem. 1985 Apr 15;148(2):213–218. doi: 10.1111/j.1432-1033.1985.tb08827.x. [DOI] [PubMed] [Google Scholar]
- Rennie P. S., Bowden J. F., Freeman S. N., Bruchovsky N., Cheng H., Lubahn D. B., Wilson E. M., French F. S., Main L. Cortisol alters gene expression during involution of the rat ventral prostate. Mol Endocrinol. 1989 Apr;3(4):703–708. doi: 10.1210/mend-3-4-703. [DOI] [PubMed] [Google Scholar]
- Rotello R. J., Hocker M. B., Gerschenson L. E. Biochemical evidence for programmed cell death in rabbit uterine epithelium. Am J Pathol. 1989 Mar;134(3):491–495. [PMC free article] [PubMed] [Google Scholar]
- Rotello R. J., Lieberman R. C., Purchio A. F., Gerschenson L. E. Coordinated regulation of apoptosis and cell proliferation by transforming growth factor beta 1 in cultured uterine epithelial cells. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3412–3415. doi: 10.1073/pnas.88.8.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandford N. L., Searle J. W., Kerr J. F. Successive waves of apoptosis in the rat prostate after repeated withdrawal of testosterone stimulation. Pathology. 1984 Oct;16(4):406–410. doi: 10.3109/00313028409084731. [DOI] [PubMed] [Google Scholar]
- Schwartz L. M., Kosz L., Kay B. K. Gene activation is required for developmentally programmed cell death. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6594–6598. doi: 10.1073/pnas.87.17.6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker N. I., Harmon B. V., Gobé G. C., Kerr J. F. Patterns of cell death. Methods Achiev Exp Pathol. 1988;13:18–54. [PubMed] [Google Scholar]
- Yuan J. Y., Horvitz H. R. The Caenorhabditis elegans genes ced-3 and ced-4 act cell autonomously to cause programmed cell death. Dev Biol. 1990 Mar;138(1):33–41. doi: 10.1016/0012-1606(90)90174-h. [DOI] [PubMed] [Google Scholar]