Abstract
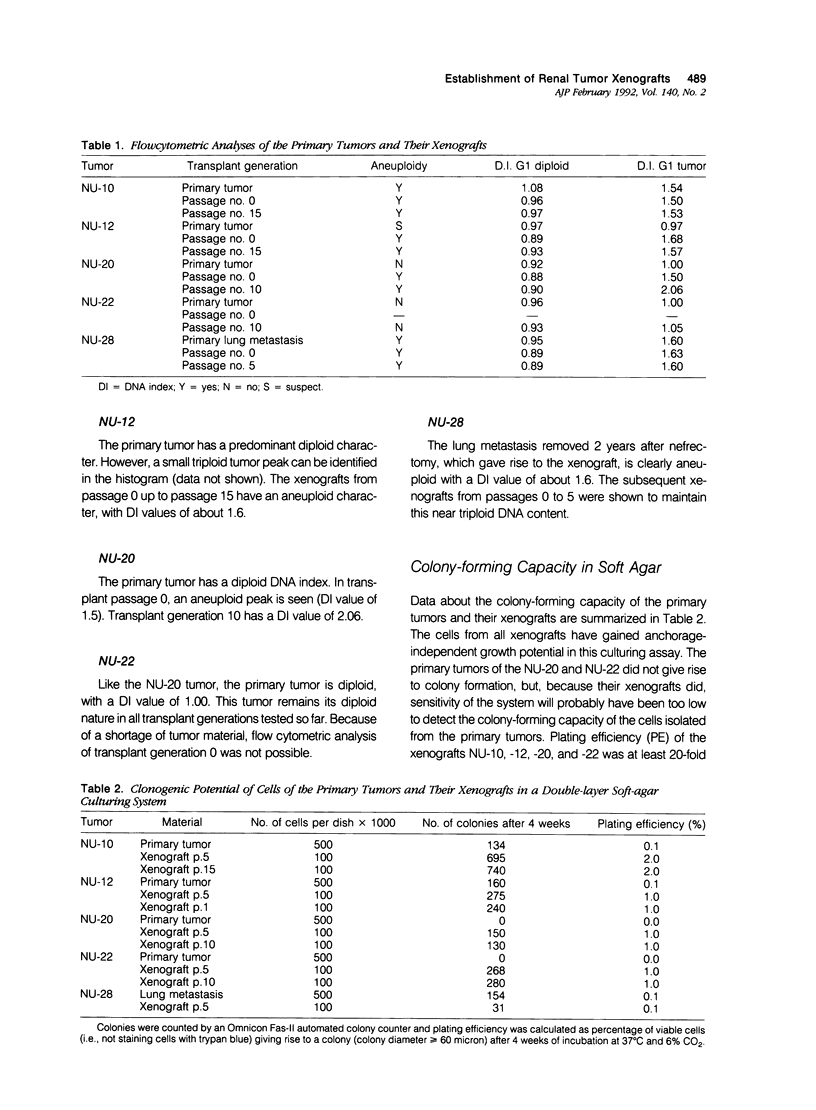
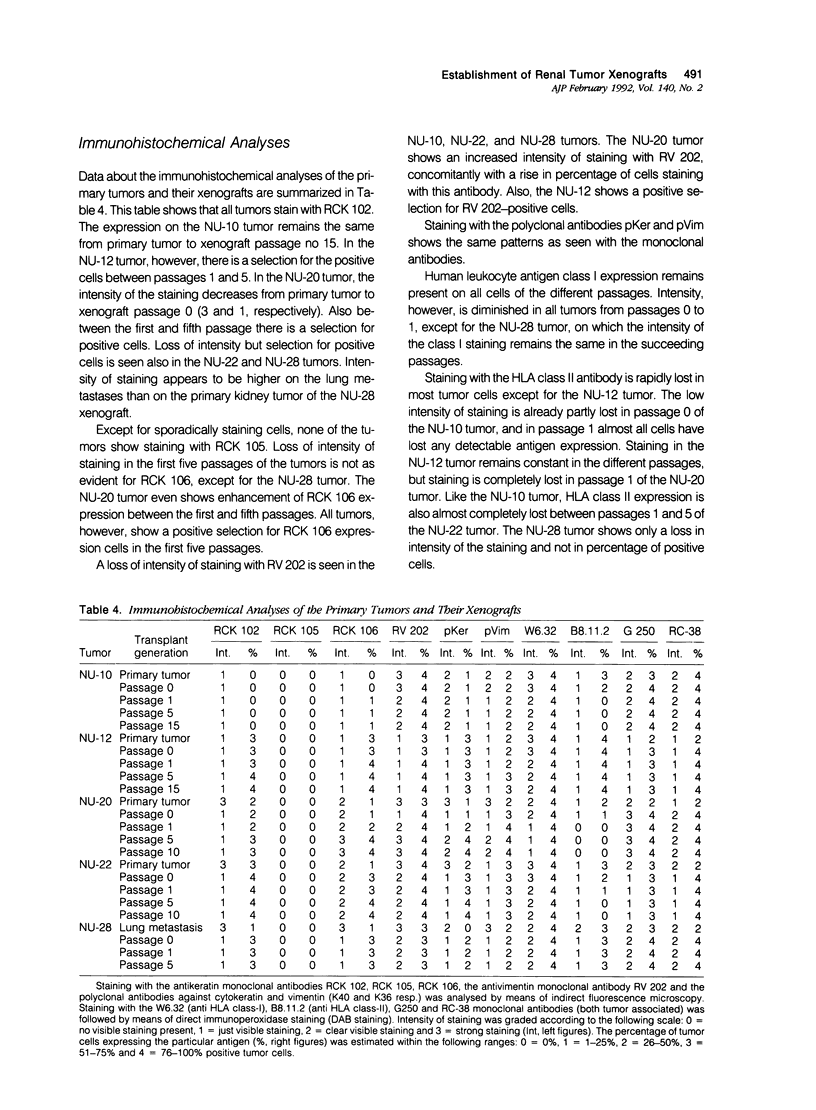
Ten different human renal cell carcinoma (RCC) primary tumors were xenografted into BALB/c nu/nu mice. Five of the tumors (NU-10, NU-12, NU-20, NU-22, and NU-28) gave rise to serially transplantable tumors that were further characterized. Histology, DNA index, immunohistochemical characteristics, growth rate, and clonogenic potential were followed from primary tumor to the 5th to 15th transplant passage. Only one of the tumors (NU-20) showed remarkable instability for all tested parameters in the first five transplant passages. Histology of the other tumors was essentially the same to the histology of the primary tumors, although differences between human and host-derived vessels were apparent. DNA index values in general showed a trend toward an aneuploid character of the xenografts. Immunohistochemical analyses showed a loss of intensity of staining but a concomitant rise in the fraction of positively staining cells with antibodies against cytokeratins, vimentin, tumor-associated antigens, and human leukocyte antigen (HLA) class I antigens. Human leukocyte antigen class II antigen expression showed a loss of intensity as well as a decrease in the fraction of positive cells. Tumor doubling time was lowest in transplant passage number 0, and stable growth was noticed in transplant passages 1 through 4. Clonogenic potential of four of the lines was higher for the xenografts than for the primary tumors. The authors conclude that, on xenografting, histologic characteristics of the primary tumor are essentially conserved. Progression in the first transplant passages, however, results in tumors with a more aggressive character.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnstable C. J., Bodmer W. F., Brown G., Galfre G., Milstein C., Williams A. F., Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell. 1978 May;14(1):9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- Beniers A. J., van Moorselaar R. J., Peelen W. P., Debruyne F. M., Schalken J. A. Differential sensitivity of renal cell carcinoma xenografts towards therapy with interferon-alpha, interferon-gamma, tumor necrosis factor and their combinations. Urol Res. 1991;19(2):91–98. doi: 10.1007/BF00368183. [DOI] [PubMed] [Google Scholar]
- Braakhuis B. J., Nauta M. M., Romijn J. C., Rutgers D. H., Smink T. Enhanced success rate of transplantation with human tumors in cyclophosphamide-treated nude mice. J Natl Cancer Inst. 1986 Feb;76(2):241–245. [PubMed] [Google Scholar]
- Clayman R. V., Figenshau R. S., Bear A., Limas C. Transplantation of human renal carcinomas into athymic mice. Cancer Res. 1985 Jun;45(6):2650–2653. [PubMed] [Google Scholar]
- Flanagan S. P. 'Nude', a new hairless gene with pleiotropic effects in the mouse. Genet Res. 1966 Dec;8(3):295–309. doi: 10.1017/s0016672300010168. [DOI] [PubMed] [Google Scholar]
- Freed S. Z., Halperin J. P., Gordon M. Idiopathic regression of metastases from renal cell carcinoma. J Urol. 1977 Oct;118(4):538–542. doi: 10.1016/s0022-5347(17)58099-4. [DOI] [PubMed] [Google Scholar]
- GRAY L. H., STEADMAN J. M. DETERMINATION OF THE OXYHAEMOGLOBIN DISSOCIATION CURVES FOR MOUSE AND RAT BLOOD. J Physiol. 1964 Dec;175:161–171. doi: 10.1113/jphysiol.1964.sp007509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habu S., Fukui H., Shimamura K., Kasai M., Nagai Y., Okumura K., Tamaoki N. In vivo effects of anti-asialo GM1. I. Reduction of NK activity and enhancement of transplanted tumor growth in nude mice. J Immunol. 1981 Jul;127(1):34–38. [PubMed] [Google Scholar]
- Hashimura T., Tubbs R. R., Connelly R., Caulfield M. J., Trindade C. S., McMahon J. T., Galetti T. P., Edinger M., Sandberg A. A., Dal Cin P. Characterization of two cell lines with distinct phenotypes and genotypes established from a patient with renal cell carcinoma. Cancer Res. 1989 Dec 15;49(24 Pt 1):7064–7071. [PubMed] [Google Scholar]
- Herman C. J., Moesker O., Kant A., Huysmans A., Vooijs G. P., Ramaekers F. C. Is renal cell (Grawitz) tumor a carcinosarcoma? Evidence from analysis of intermediate filament types. Virchows Arch B Cell Pathol Incl Mol Pathol. 1983;44(1):73–83. doi: 10.1007/BF02890161. [DOI] [PubMed] [Google Scholar]
- Hünig T., Bevan M. J. Specificity of cytotoxic T cells from athymic mice. J Exp Med. 1980 Sep 1;152(3):688–702. doi: 10.1084/jem.152.3.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain R. K. Determinants of tumor blood flow: a review. Cancer Res. 1988 May 15;48(10):2641–2658. [PubMed] [Google Scholar]
- Lauk S., Zietman A., Skates S., Fabian R., Suit H. D. Comparative morphometric study of tumor vasculature in human squamous cell carcinomas and their xenotransplants in athymic nude mice. Cancer Res. 1989 Aug 15;49(16):4557–4561. [PubMed] [Google Scholar]
- Lefrançois D., Olschwang S., Delattre O., Muleris M., Dutrillaux A. M., Thomas G., Dutrillaux B. Preservation of chromosome and DNA characteristics of human colorectal adenocarcinomas after passage in nude mice. Int J Cancer. 1989 Nov 15;44(5):871–878. doi: 10.1002/ijc.2910440521. [DOI] [PubMed] [Google Scholar]
- Lemonnier F. A., Rebai N., Le Bouteiller P. P., Malissen B., Caillol D. H., Kourilsky F. M. Epitopic analysis of detergent-solubilized HLA molecules by solid-phase radioimmunoassay. J Immunol Methods. 1982 Oct 15;54(1):9–22. doi: 10.1016/0022-1759(82)90108-9. [DOI] [PubMed] [Google Scholar]
- Ljungberg B., Stenling R., Roos G. Flow cytometric DNA analysis of renal-cell carcinoma. A study of fine needle aspiration biopsies in comparison with multiple surgical samples. Anal Quant Cytol Histol. 1987 Dec;9(6):505–508. [PubMed] [Google Scholar]
- Naito S., von Eschenbach A. C., Giavazzi R., Fidler I. J. Growth and metastasis of tumor cells isolated from a human renal cell carcinoma implanted into different organs of nude mice. Cancer Res. 1986 Aug;46(8):4109–4115. [PubMed] [Google Scholar]
- Oosterwijk E., Ruiter D. J., Wakka J. C., Huiskens-van der Meij J. W., Jonas U., Fleuren G. J., Zwartendijk J., Hoedemaeker P., Warnaar S. O. Immunohistochemical analysis of monoclonal antibodies to renal antigens. Application in the diagnosis of renal cell carcinoma. Am J Pathol. 1986 May;123(2):301–309. [PMC free article] [PubMed] [Google Scholar]
- Pantelouris E. M. Absence of thymus in a mouse mutant. Nature. 1968 Jan 27;217(5126):370–371. doi: 10.1038/217370a0. [DOI] [PubMed] [Google Scholar]
- Pantelouris E. M. Absence of thymus in a mouse mutant. Nature. 1968 Jan 27;217(5126):370–371. doi: 10.1038/217370a0. [DOI] [PubMed] [Google Scholar]
- Piontek G. E., Taniguchi K., Ljunggren H. G., Grönberg A., Kiessling R., Klein G., Kärre K. YAC-1 MHC class I variants reveal an association between decreased NK sensitivity and increased H-2 expression after interferon treatment or in vivo passage. J Immunol. 1985 Dec;135(6):4281–4288. [PubMed] [Google Scholar]
- Ramaekers F. C., Puts J. J., Moesker O., Kant A., Huysmans A., Haag D., Jap P. H., Herman C. J., Vooijs G. P. Antibodies to intermediate filament proteins in the immunohistochemical identification of human tumours: an overview. Histochem J. 1983 Jul;15(7):691–713. doi: 10.1007/BF01002988. [DOI] [PubMed] [Google Scholar]
- Ramaekers F., Huysmans A., Schaart G., Moesker O., Vooijs P. Tissue distribution of keratin 7 as monitored by a monoclonal antibody. Exp Cell Res. 1987 May;170(1):235–249. doi: 10.1016/0014-4827(87)90133-9. [DOI] [PubMed] [Google Scholar]
- Ramaekers F., van Niekerk C., Poels L., Schaafsma E., Huijsmans A., Robben H., Schaart G., Vooijs P. Use of monoclonal antibodies to keratin 7 in the differential diagnosis of adenocarcinomas. Am J Pathol. 1990 Mar;136(3):641–655. [PMC free article] [PubMed] [Google Scholar]
- Solesvik O. V., Rofstad E. K., Brustad T. Vascular structure of five human malignant melanomas grown in athymic nude mice. Br J Cancer. 1982 Oct;46(4):557–567. doi: 10.1038/bjc.1982.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoenes W., Störkel S., Rumpelt H. J. Histopathology and classification of renal cell tumors (adenomas, oncocytomas and carcinomas). The basic cytological and histopathological elements and their use for diagnostics. Pathol Res Pract. 1986 May;181(2):125–143. doi: 10.1016/S0344-0338(86)80001-2. [DOI] [PubMed] [Google Scholar]
- Verheijen R. H., Feitz W. F., Beck J. L., Debruyne F. M., Vooys G. P., Kenemans P., Herman C. J. Cell DNA content--correlation with clonogenicity in the human tumour cloning system (HTCS). Int J Cancer. 1985 May 15;35(5):653–657. doi: 10.1002/ijc.2910350514. [DOI] [PubMed] [Google Scholar]
- Watanabe S., Shimosato Y., Kuroki M., Sato Y., Nakajima T. Transplantability of human lymphoid cell line, lymphoma, and leukemia in splenectomized and/or irradiated nude mice. Cancer Res. 1980 Jul;40(7):2588–2595. [PubMed] [Google Scholar]
- van Muijen G. N., Cornelissen I. M., Jansen C. F., Ruiter D. J. Progression markers in metastasizing human melanoma cells xenografted to nude mice (review). Anticancer Res. 1989 Jul-Aug;9(4):879–884. [PubMed] [Google Scholar]



