Abstract
Alpha B-crystallin, a major protein of the vertebrate lens, is found in the central nervous system (CNS) and is a major protein component of Rosenthal fibers (RF), intracytoplasmic inclusions within astrocytes. Its level of expression in the normal CNS is low and appears to be confined to glial cells, both astrocytes and oligodendrocytes. A number of human brains displaying a variety of pathologic changes were examined by immunohistochemistry with an anti-alpha B-crystallin antiserum and increased immunoreactivity was found in astrocytes and oligodendrocytes without the formation of RFs. Furthermore, some neurons in neurodegenerative disorders were also immunolabeled with the anti-alpha B-crystallin antiserum. Thus, the accumulation of alpha B-crystallin appears to be part of the repertoire of reactive processes of CNS glial cells and some neurons in pathologic conditions.
Full text
PDF


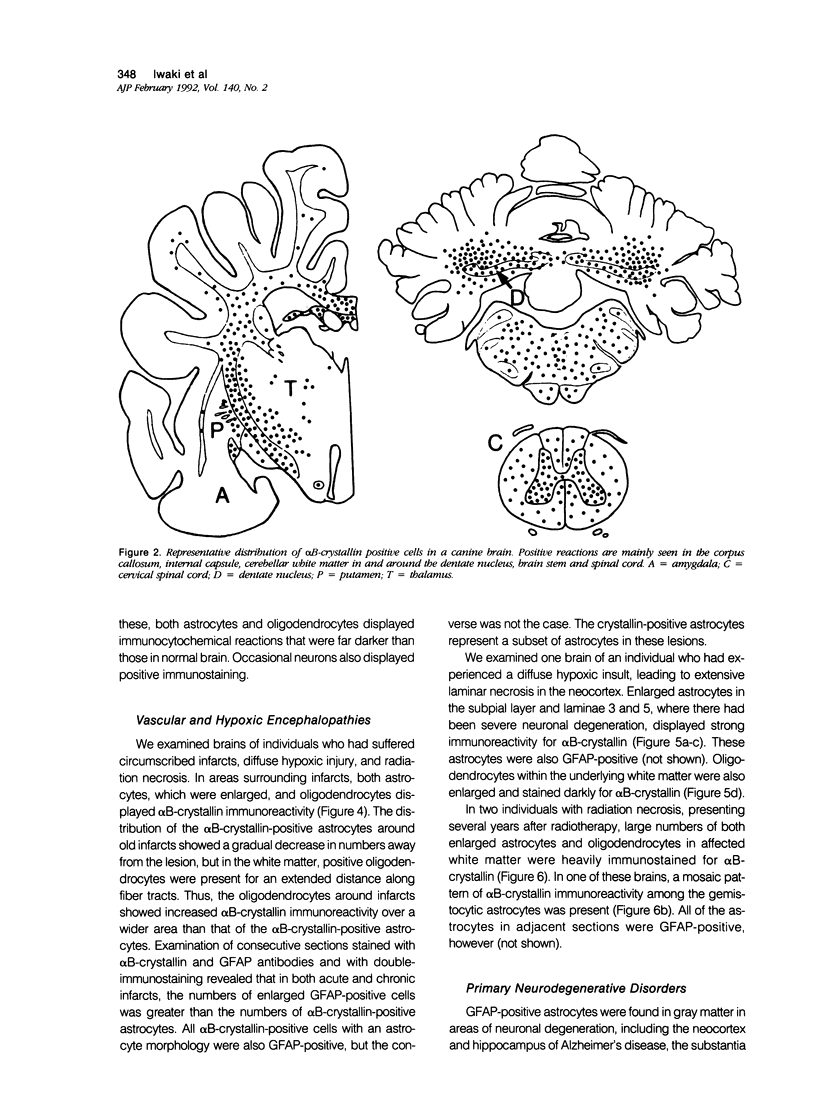
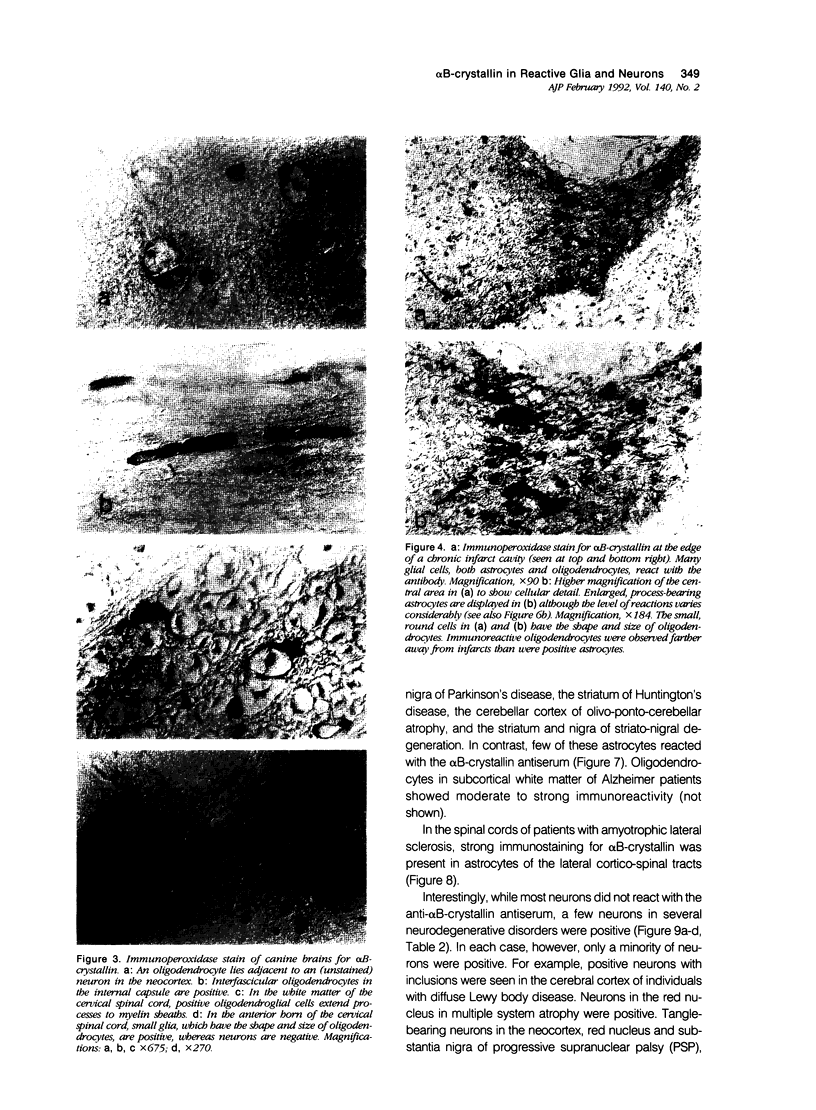
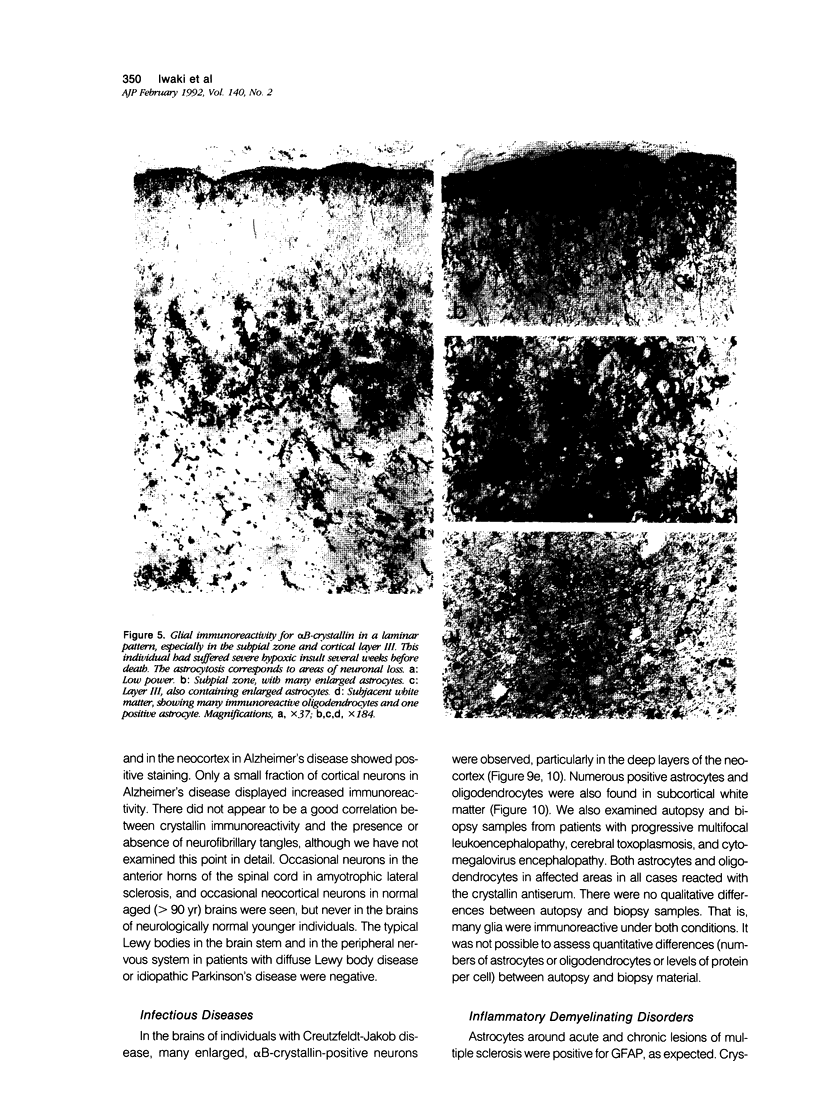


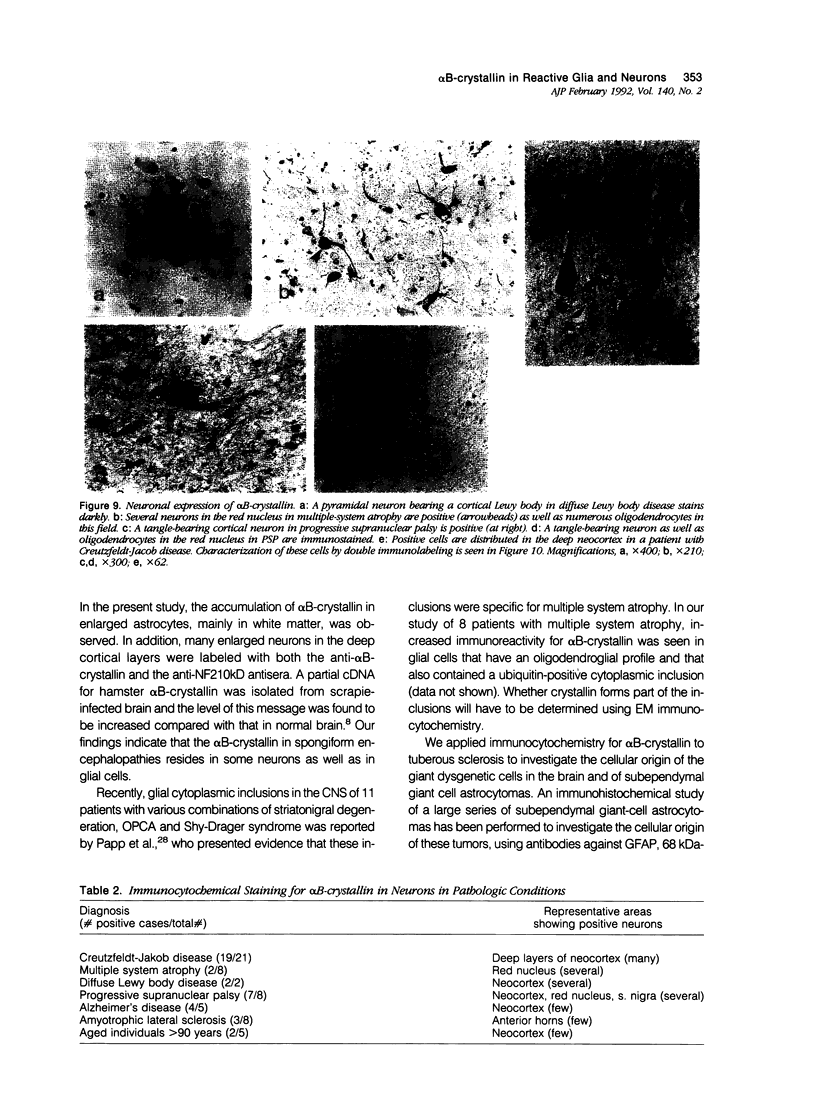


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amaducci L., Forno K. I., Eng L. F. Glial fibrillary acidic protein in cryogenic lesions of the rat brain. Neurosci Lett. 1981 Jan 1;21(1):27–32. doi: 10.1016/0304-3940(81)90052-5. [DOI] [PubMed] [Google Scholar]
- Bhat S. P., Nagineni C. N. alpha B subunit of lens-specific protein alpha-crystallin is present in other ocular and non-ocular tissues. Biochem Biophys Res Commun. 1989 Jan 16;158(1):319–325. doi: 10.1016/s0006-291x(89)80215-3. [DOI] [PubMed] [Google Scholar]
- Bonnin J. M., Rubinstein L. J., Papasozomenos S. C., Marangos P. J. Subependymal giant cell astrocytoma. Significance and possible cytogenetic implications of an immunohistochemical study. Acta Neuropathol. 1984;62(3):185–193. doi: 10.1007/BF00691851. [DOI] [PubMed] [Google Scholar]
- Borrett D., Becker L. E. Alexander's disease. A disease of astrocytes. Brain. 1985 Jun;108(Pt 2):367–385. doi: 10.1093/brain/108.2.367. [DOI] [PubMed] [Google Scholar]
- Dubin R. A., Ally A. H., Chung S., Piatigorsky J. Human alpha B-crystallin gene and preferential promoter function in lens. Genomics. 1990 Aug;7(4):594–601. doi: 10.1016/0888-7543(90)90204-8. [DOI] [PubMed] [Google Scholar]
- Dubin R. A., Wawrousek E. F., Piatigorsky J. Expression of the murine alpha B-crystallin gene is not restricted to the lens. Mol Cell Biol. 1989 Mar;9(3):1083–1091. doi: 10.1128/mcb.9.3.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duguid J. R., Rohwer R. G., Seed B. Isolation of cDNAs of scrapie-modulated RNAs by subtractive hybridization of a cDNA library. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5738–5742. doi: 10.1073/pnas.85.15.5738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman J. E., Chiu F. C. Growth kinetics, cell shape, and the cytoskeleton of primary astrocyte cultures. J Neurochem. 1984 Jan;42(1):175–184. doi: 10.1111/j.1471-4159.1984.tb09714.x. [DOI] [PubMed] [Google Scholar]
- Goldman J. E., Corbin E. Isolation of a major protein component of Rosenthal fibers. Am J Pathol. 1988 Mar;130(3):569–578. [PMC free article] [PubMed] [Google Scholar]
- Goldman J. E., Corbin E. Rosenthal fibers contain ubiquitinated alpha B-crystallin. Am J Pathol. 1991 Oct;139(4):933–938. [PMC free article] [PubMed] [Google Scholar]
- Guesdon J. L., Ternynck T., Avrameas S. The use of avidin-biotin interaction in immunoenzymatic techniques. J Histochem Cytochem. 1979 Aug;27(8):1131–1139. doi: 10.1177/27.8.90074. [DOI] [PubMed] [Google Scholar]
- Herndon R. M., Rubinstein L. J., Freeman J. M., Mathieson G. Light and electron microscopic observations on Rosenthal fibers in Alexander's disease and in multiple sclerosis. J Neuropathol Exp Neurol. 1970 Oct;29(4):524–551. doi: 10.1097/00005072-197010000-00002. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Soban E. Color modification of diaminobenzidine (DAB) precipitation by metallic ions and its application for double immunohistochemistry. J Histochem Cytochem. 1982 Oct;30(10):1079–1082. doi: 10.1177/30.10.6182185. [DOI] [PubMed] [Google Scholar]
- Ingolia T. D., Craig E. A. Four small Drosophila heat shock proteins are related to each other and to mammalian alpha-crystallin. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2360–2364. doi: 10.1073/pnas.79.7.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaki A., Iwaki T., Goldman J. E., Liem R. K. Multiple mRNAs of rat brain alpha-crystallin B chain result from alternative transcriptional initiation. J Biol Chem. 1990 Dec 25;265(36):22197–22203. [PubMed] [Google Scholar]
- Iwaki T., Fukui M., Takeshita I., Tsuneyoshi M., Tateishi J. Hemangiopericytoma of the meninges: a clinicopathologic and immunohistochemical study. Clin Neuropathol. 1988 May-Jun;7(3):93–99. [PubMed] [Google Scholar]
- Iwaki T., Kume-Iwaki A., Goldman J. E. Cellular distribution of alpha B-crystallin in non-lenticular tissues. J Histochem Cytochem. 1990 Jan;38(1):31–39. doi: 10.1177/38.1.2294148. [DOI] [PubMed] [Google Scholar]
- Iwaki T., Kume-Iwaki A., Liem R. K., Goldman J. E. Alpha B-crystallin is expressed in non-lenticular tissues and accumulates in Alexander's disease brain. Cell. 1989 Apr 7;57(1):71–78. doi: 10.1016/0092-8674(89)90173-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- LeVine S. M., Macklin W. B. Biotin enrichment in oligodendrocytes in the rat brain. Brain Res. 1988 Mar 15;444(1):199–203. doi: 10.1016/0006-8993(88)90930-4. [DOI] [PubMed] [Google Scholar]
- Lee V. M., Carden M. J., Trojanowski J. Q. Novel monoclonal antibodies provide evidence for the in situ existence of a nonphosphorylated form of the largest neurofilament subunit. J Neurosci. 1986 Mar;6(3):850–858. doi: 10.1523/JNEUROSCI.06-03-00850.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe J., Landon M., Pike I., Spendlove I., McDermott H., Mayer R. J. Dementia with beta-amyloid deposition: involvement of alpha B-crystallin supports two main diseases. Lancet. 1990 Aug 25;336(8713):515–516. doi: 10.1016/0140-6736(90)92075-s. [DOI] [PubMed] [Google Scholar]
- Ludwin S. K. Proliferation of mature oligodendrocytes after trauma to the central nervous system. Nature. 1984 Mar 15;308(5956):274–275. doi: 10.1038/308274a0. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Nakazato Y., Hirato J., Ishida Y., Hoshi S., Hasegawa M., Fukuda T. Swollen cortical neurons in Creutzfeldt-Jakob disease contain a phosphorylated neurofilament epitope. J Neuropathol Exp Neurol. 1990 May;49(3):197–205. doi: 10.1097/00005072-199005000-00001. [DOI] [PubMed] [Google Scholar]
- Ogasawara N. Multiple Sklerose mit Rosenthalschen Fasern. Acta Neuropathol. 1965 Oct 4;5(1):61–68. doi: 10.1007/BF00689163. [DOI] [PubMed] [Google Scholar]
- Papp M. I., Kahn J. E., Lantos P. L. Glial cytoplasmic inclusions in the CNS of patients with multiple system atrophy (striatonigral degeneration, olivopontocerebellar atrophy and Shy-Drager syndrome). J Neurol Sci. 1989 Dec;94(1-3):79–100. doi: 10.1016/0022-510x(89)90219-0. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J., Wistow G. J. Enzyme/crystallins: gene sharing as an evolutionary strategy. Cell. 1989 Apr 21;57(2):197–199. doi: 10.1016/0092-8674(89)90956-2. [DOI] [PubMed] [Google Scholar]
- Prineas J. W., Kwon E. E., Goldenberg P. Z., Ilyas A. A., Quarles R. H., Benjamins J. A., Sprinkle T. J. Multiple sclerosis. Oligodendrocyte proliferation and differentiation in fresh lesions. Lab Invest. 1989 Nov;61(5):489–503. [PubMed] [Google Scholar]
- Sawa H., Takeshita I., Kuramitsu M., Mannoji H., Machi T., Fukui M., Kitamura K. Neuronal and glial proteins in medulloblastomas. I. Immunohistochemical study. Anticancer Res. 1986 Sep-Oct;6(5):905–909. [PubMed] [Google Scholar]
- Shafit-Zagardo B., Peterson C., Goldman J. E. Rapid increases in glial fibrillary acidic protein mRNA and protein levels in the copper-deficient, brindled mouse. J Neurochem. 1988 Oct;51(4):1258–1266. doi: 10.1111/j.1471-4159.1988.tb03095.x. [DOI] [PubMed] [Google Scholar]
- Sternberger L. A., Sternberger N. H. Monoclonal antibodies distinguish phosphorylated and nonphosphorylated forms of neurofilaments in situ. Proc Natl Acad Sci U S A. 1983 Oct;80(19):6126–6130. doi: 10.1073/pnas.80.19.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomokane N., Iwaki T., Tateishi J., Iwaki A., Goldman J. E. Rosenthal fibers share epitopes with alpha B-crystallin, glial fibrillary acidic protein, and ubiquitin, but not with vimentin. Immunoelectron microscopy with colloidal gold. Am J Pathol. 1991 Apr;138(4):875–885. [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong W. W., Leunissen J. A., Leenen P. J., Zweers A., Versteeg M. Dogfish alpha-crystallin sequences. Comparison with small heat shock proteins and Schistosoma egg antigen. J Biol Chem. 1988 Apr 15;263(11):5141–5149. [PubMed] [Google Scholar]












