Abstract
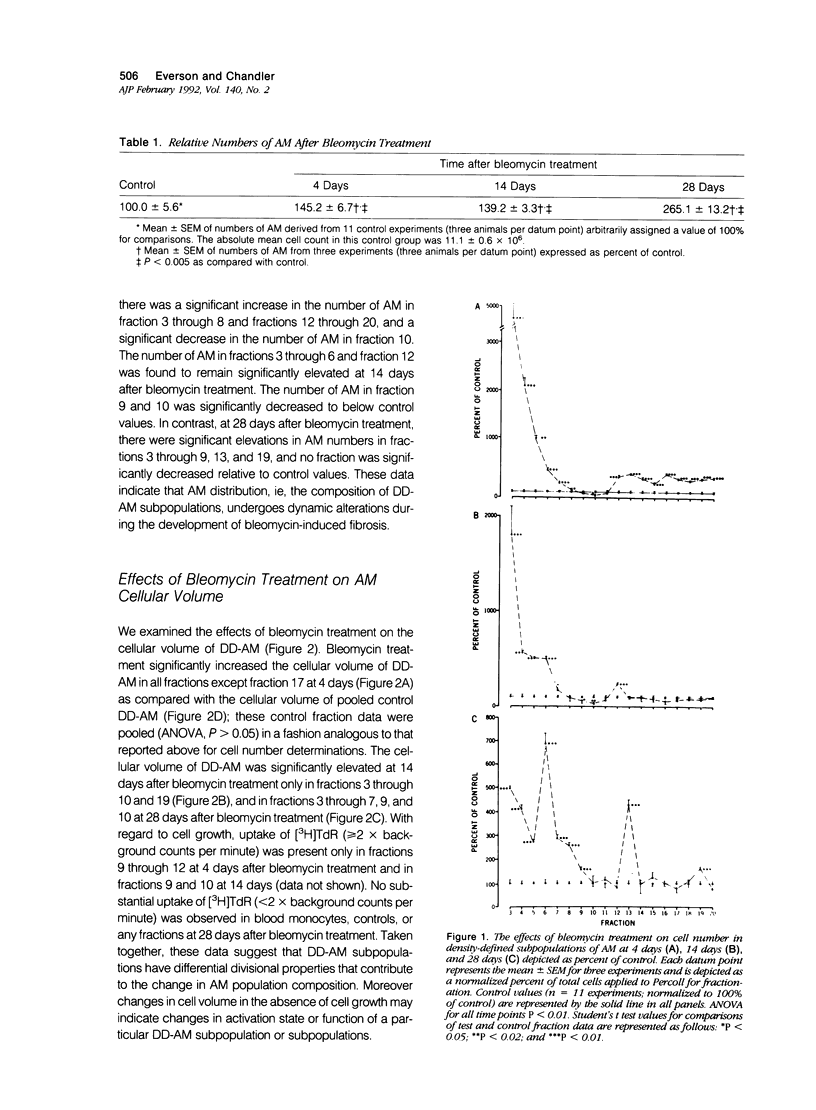
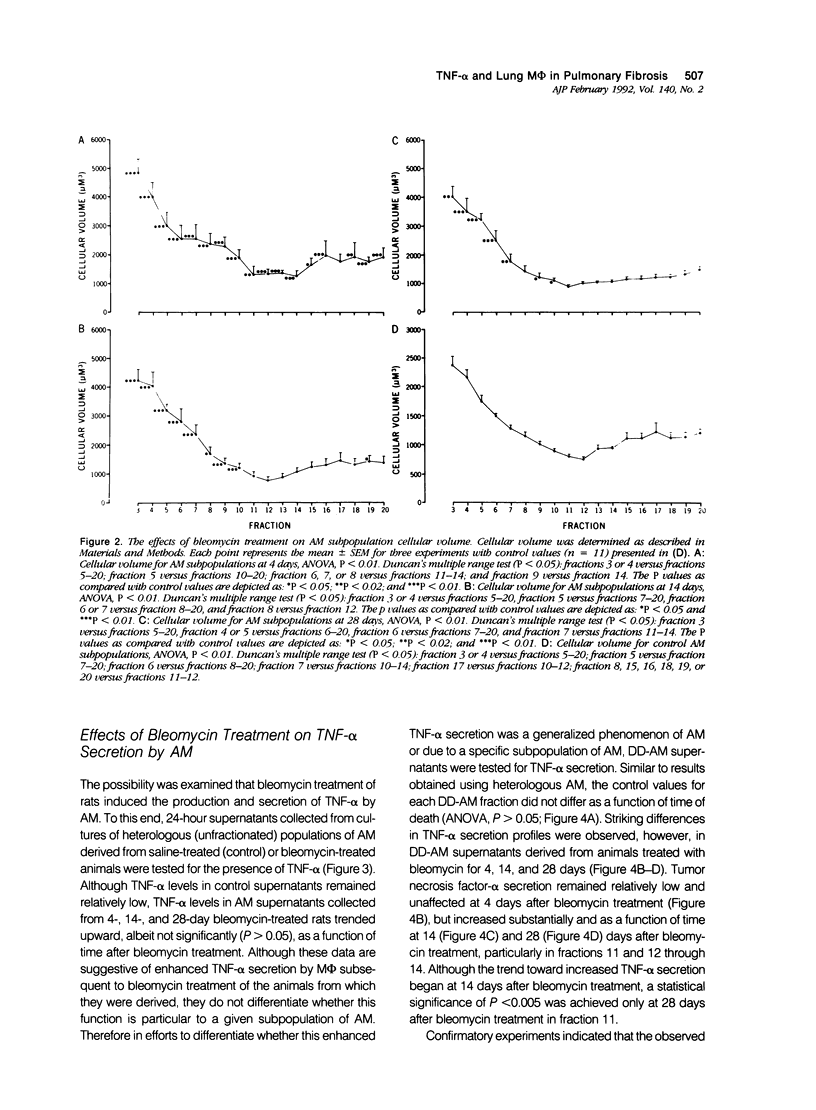
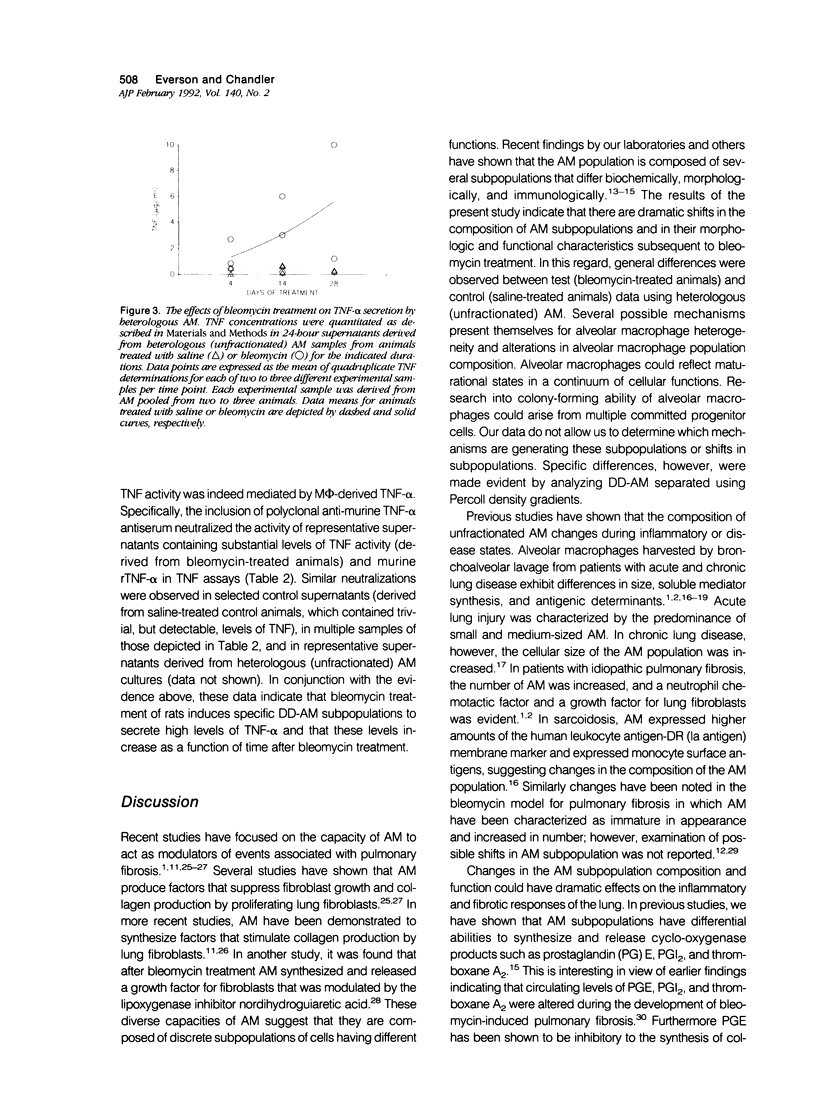
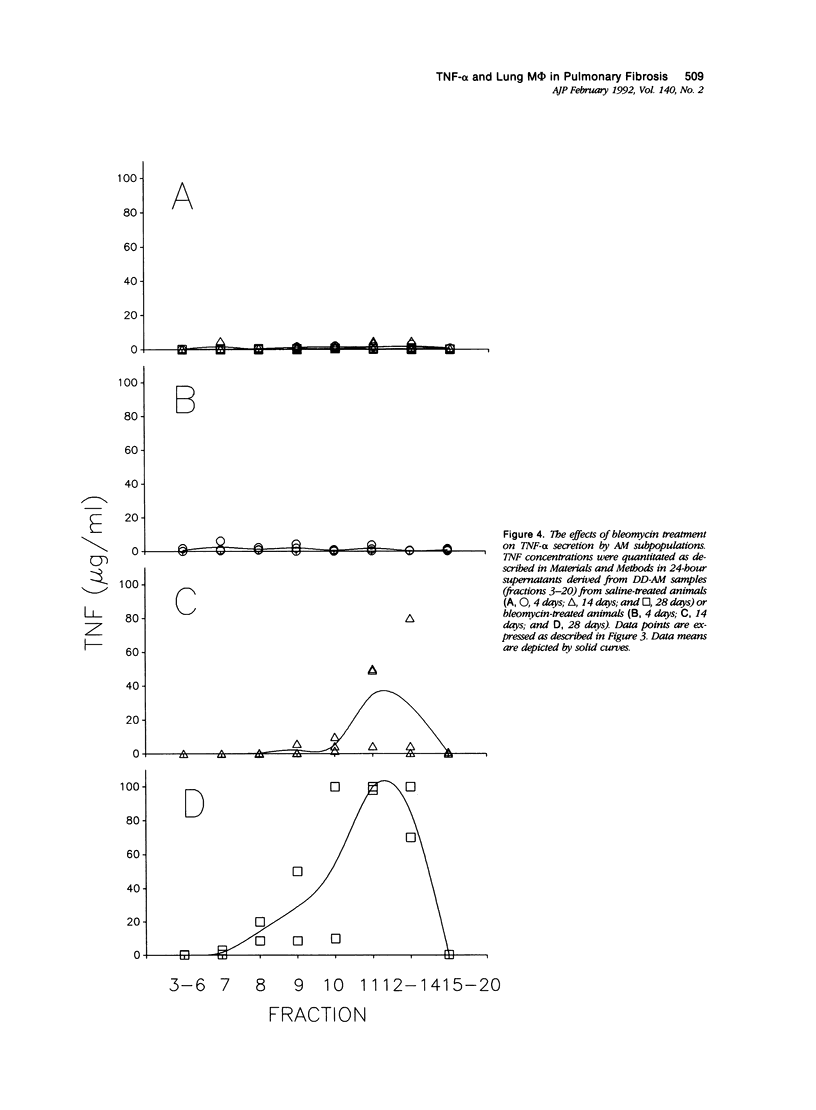
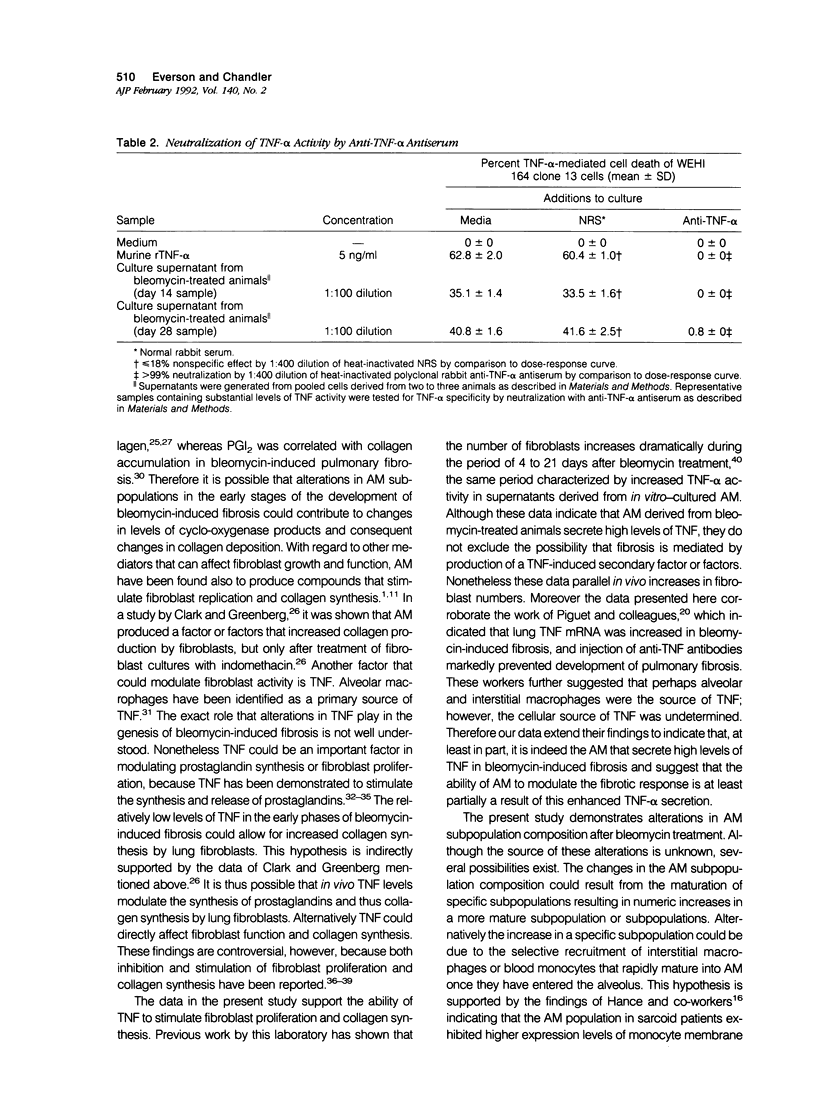
Previous studies indicate that heterogeneous alveolar macrophages (AM) play a pivotal role in events associated with bleomycin-induced pulmonary fibrosis. A critical role has been suggested for tumor necrosis factor-alpha (TNF-alpha), a product of activated macrophages, in this fibrotic process. The present study examined whether the characteristics and function (TNF-alpha secretion) of rat AM subpopulations were altered during the development of bleomycin-induced fibrosis. After intratracheal bleomycin treatment, AM were separated into 18 density-defined subpopulations. Bleomycin treatment altered the distribution and morphology of AM subpopulations of densities 1.017 to 1.061 g/ml at all time points studied (4, 14, and 28 days). Subpopulations of densities 1.090 to 1.141 g/ml were affected only at 4 days after bleomycin treatment. Tumor necrosis factor-alpha secretion increased with time in 14- and 28-day samples of bleomycin-treated rats, particularly in subpopulations of densities 1.075 to 1.097 g/ml. These data indicate that alterations in the distribution, morphology, and function of AM subpopulations accompany the development of bleomycin-induced pulmonary fibrosis. When coupled with previous studies suggesting that TNF-alpha plays a role in the fibrotic process in this disease model, these data indicate that AM of densities 1.075 to 1.097 g/ml are responsible for the production of TNF-alpha associated with bleomycin-induced pulmonary fibrosis.
Full text
PDF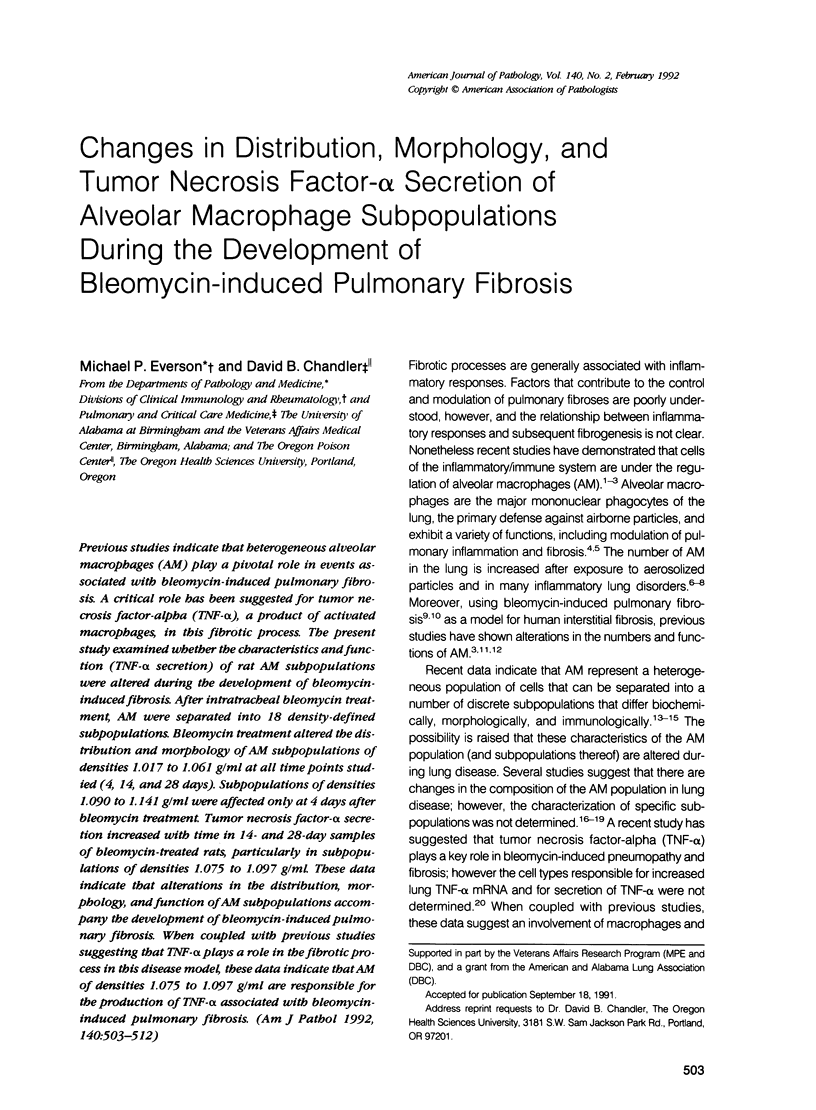




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachwich P. R., Chensue S. W., Larrick J. W., Kunkel S. L. Tumor necrosis factor stimulates interleukin-1 and prostaglandin E2 production in resting macrophages. Biochem Biophys Res Commun. 1986 Apr 14;136(1):94–101. doi: 10.1016/0006-291x(86)90881-8. [DOI] [PubMed] [Google Scholar]
- Bitterman P. B., Adelberg S., Crystal R. G. Mechanisms of pulmonary fibrosis. Spontaneous release of the alveolar macrophage-derived growth factor in the interstitial lung disorders. J Clin Invest. 1983 Nov;72(5):1801–1813. doi: 10.1172/JCI111140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Chandler D. B., Fuller W. C., Jackson R. M., Fulmer J. D. Fractionation of rat alveolar macrophages by isopycnic centrifugation: morphological, cytochemical, biochemical, and functional properties. J Leukoc Biol. 1986 Apr;39(4):371–383. doi: 10.1002/jlb.39.4.371. [DOI] [PubMed] [Google Scholar]
- Chandler D. B., Fuller W. C., Jackson R. M., Fulmer J. D. Studies of membrane receptors and phagocytosis in subpopulations of rat alveolar macrophages. Am Rev Respir Dis. 1986 Mar;133(3):461–467. doi: 10.1164/arrd.1986.133.3.461. [DOI] [PubMed] [Google Scholar]
- Chandler D. B., Fulmer J. D. Prostaglandin synthesis and release by subpopulations of rat alveolar macrophages. J Immunol. 1987 Aug 1;139(3):893–898. [PubMed] [Google Scholar]
- Chandler D. B., Giri S. N. Changes in plasma concentrations of prostaglandins and plasma angiotensin-converting enzyme during bleomycin-induced lung fibrosis in hamsters. Am Rev Respir Dis. 1983 Jul;128(1):71–76. doi: 10.1164/arrd.1983.128.1.71. [DOI] [PubMed] [Google Scholar]
- Chandler D. B., Hyde D. M., Giri S. N. Morphometric estimates of infiltrative cellular changes during the development of bleomycin-induced pulmonary fibrosis in hamsters. Am J Pathol. 1983 Aug;112(2):170–177. [PMC free article] [PubMed] [Google Scholar]
- Clark J. G., Greenberg J. Modulation of the effects of alveolar macrophages on lung fibroblast collagen production rate. Am Rev Respir Dis. 1987 Jan;135(1):52–56. doi: 10.1164/arrd.1987.135.1.52. [DOI] [PubMed] [Google Scholar]
- Clark J. G., Kostal K. M., Marino B. A. Bleomycin-induced pulmonary fibrosis in hamsters. An alveolar macrophage product increases fibroblast prostaglandin E2 and cyclic adenosine monophosphate and suppresses fibroblast proliferation and collagen production. J Clin Invest. 1983 Dec;72(6):2082–2091. doi: 10.1172/JCI111173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniele R. P., Dauber J. H., Rossman M. D. Immunologic abnormalities in sarcoidosis. Ann Intern Med. 1980 Mar;92(3):406–416. doi: 10.7326/0003-4819-92-3-406. [DOI] [PubMed] [Google Scholar]
- Dayer J. M., Beutler B., Cerami A. Cachectin/tumor necrosis factor stimulates collagenase and prostaglandin E2 production by human synovial cells and dermal fibroblasts. J Exp Med. 1985 Dec 1;162(6):2163–2168. doi: 10.1084/jem.162.6.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker T., Lohmann-Matthes M. L., Gifford G. E. Cell-associated tumor necrosis factor (TNF) as a killing mechanism of activated cytotoxic macrophages. J Immunol. 1987 Feb 1;138(3):957–962. [PubMed] [Google Scholar]
- Elias J. A., Gustilo K., Freundlich B. Human alveolar macrophage and blood monocyte inhibition of fibroblast proliferation. Evidence for synergy between interleukin-1 and tumor necrosis factor. Am Rev Respir Dis. 1988 Dec;138(6):1595–1603. doi: 10.1164/ajrccm/138.6.1595. [DOI] [PubMed] [Google Scholar]
- Elias J. A., Rossman M. D., Zurier R. B., Daniele R. P. Human alveolar macrophage inhibition of lung fibroblast growth. A prostaglandin-dependent process. Am Rev Respir Dis. 1985 Jan;131(1):94–99. doi: 10.1164/arrd.1985.131.1.94. [DOI] [PubMed] [Google Scholar]
- Elias J. A. Tumor necrosis factor interacts with interleukin-1 and interferons to inhibit fibroblast proliferation via fibroblast prostaglandin-dependent and -independent mechanisms. Am Rev Respir Dis. 1988 Sep;138(3):652–658. doi: 10.1164/ajrccm/138.3.652. [DOI] [PubMed] [Google Scholar]
- Espevik T., Nissen-Meyer J. A highly sensitive cell line, WEHI 164 clone 13, for measuring cytotoxic factor/tumor necrosis factor from human monocytes. J Immunol Methods. 1986 Dec 4;95(1):99–105. doi: 10.1016/0022-1759(86)90322-4. [DOI] [PubMed] [Google Scholar]
- Hance A. J., Douches S., Winchester R. J., Ferrans V. J., Crystal R. G. Characterization of mononuclear phagocyte subpopulations in the human lung by using monoclonal antibodies: changes in alveolar macrophage phenotype associated with pulmonary sarcoidosis. J Immunol. 1985 Jan;134(1):284–292. [PubMed] [Google Scholar]
- Hocking W. G., Golde D. W. The pulmonary-alveolar macrophage (first of two parts). N Engl J Med. 1979 Sep 13;301(11):580–587. doi: 10.1056/NEJM197909133011104. [DOI] [PubMed] [Google Scholar]
- Hocking W. G., Golde D. W. The pulmonary-alveolar macrophage (second of two parts). N Engl J Med. 1979 Sep 20;301(12):639–645. doi: 10.1056/NEJM197909203011205. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Gadek J. E., Fales H. M., Crystal R. G. Human alveolar macrophage-derived chemotactic factor for neutrophils. Stimuli and partial characterization. J Clin Invest. 1980 Sep;66(3):473–483. doi: 10.1172/JCI109878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunninghake G. W., Kawanami O., Ferrans V. J., Young R. C., Jr, Roberts W. C., Crystal R. G. Characterization of the inflammatory and immune effector cells in the lung parenchyma of patients with interstitial lung disease. Am Rev Respir Dis. 1981 Apr;123(4 Pt 1):407–412. doi: 10.1164/arrd.1981.123.4.407. [DOI] [PubMed] [Google Scholar]
- Jalkanen M., Penttinen R. Enhanced fibroblast collagen production by a macrophage-derived factor (CEMF). Biochem Biophys Res Commun. 1982 Sep 30;108(2):447–453. doi: 10.1016/0006-291x(82)90849-x. [DOI] [PubMed] [Google Scholar]
- Kaelin R. M., Center D. M., Bernardo J., Grant M., Snider G. L. The role of macrophage-derived chemoattractant activities in the early inflammatory events of bleomycin-induced pulmonary injury. Am Rev Respir Dis. 1983 Jul;128(1):132–137. doi: 10.1164/arrd.1983.128.1.132. [DOI] [PubMed] [Google Scholar]
- Kahaleh M. B., DeLustro F., Bock W., LeRoy E. C. Human monocyte modulation of endothelial cells and fibroblast growth: possible mechanism for fibrosis. Clin Immunol Immunopathol. 1986 May;39(2):242–255. doi: 10.1016/0090-1229(86)90088-7. [DOI] [PubMed] [Google Scholar]
- Korn J. H., Halushka P. V., LeRoy E. C. Mononuclear cell modulation of connective tissue function: suppression of fibroblast growth by stimulation of endogenous prostaglandin production. J Clin Invest. 1980 Feb;65(2):543–554. doi: 10.1172/JCI109698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs E. J., Kelley J. Secretion of macrophage-derived growth factor during acute lung injury induced by bleomycin. J Leukoc Biol. 1985 Jan;37(1):1–14. doi: 10.1002/jlb.37.1.1. [DOI] [PubMed] [Google Scholar]
- Lenzini L., Heather C. J., Rottoli L., Rottoli P. Studies on bronchoalveolar cells in humans. I. Preliminary morphological studies in various respiratory diseases. Respiration. 1978;36(3):145–152. doi: 10.1159/000193939. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Fong T. A. Specific assays for cytokine production by T cells. J Immunol Methods. 1989 Jan 17;116(2):151–158. doi: 10.1016/0022-1759(89)90198-1. [DOI] [PubMed] [Google Scholar]
- Phan S. H., Kunkel S. L. Inhibition of bleomycin-induced pulmonary fibrosis by nordihydroguaiaretic acid. The role of alveolar macrophage activation and mediator production. Am J Pathol. 1986 Aug;124(2):343–352. [PMC free article] [PubMed] [Google Scholar]
- Piguet P. F., Collart M. A., Grau G. E., Kapanci Y., Vassalli P. Tumor necrosis factor/cachectin plays a key role in bleomycin-induced pneumopathy and fibrosis. J Exp Med. 1989 Sep 1;170(3):655–663. doi: 10.1084/jem.170.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postlethwaite A. E., Smith G. N., Mainardi C. L., Seyer J. M., Kang A. H. Lymphocyte modulation of fibroblast function in vitro: stimulation and inhibition of collagen production by different effector molecules. J Immunol. 1984 May;132(5):2470–2477. [PubMed] [Google Scholar]
- Razma A. G., Lynch J. P., 3rd, Wilson B. S., Ward P. A., Kunkel S. L. Expression of Ia-like (DR) antigen on human alveolar macrophages isolated by bronchoalveolar lavage. Am Rev Respir Dis. 1984 Mar;129(3):419–424. doi: 10.1164/arrd.1984.129.3.419. [DOI] [PubMed] [Google Scholar]
- Rennard S. I., Hunninghake G. W., Bitterman P. B., Crystal R. G. Production of fibronectin by the human alveolar macrophage: mechanism for the recruitment of fibroblasts to sites of tissue injury in interstitial lung diseases. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7147–7151. doi: 10.1073/pnas.78.11.7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider G. L., Celli B. R., Goldstein R. H., O'Brien J. J., Lucey E. C. Chronic interstitial pulmonary fibrosis produced in hamsters by endotracheal bleomycin. Lung volumes, volume-pressure relations, carbon monoxide uptake, and arterial blood gas studied. Am Rev Respir Dis. 1978 Feb;117(2):289–297. doi: 10.1164/arrd.1978.117.2.289. [DOI] [PubMed] [Google Scholar]
- Snider G. L., Hayes J. A., Korthy A. L. Chronic interstitial pulmonary fibrosis produced in hamsters by endotracheal bleomycin: pathology and stereology. Am Rev Respir Dis. 1978 Jun;117(6):1099–1108. doi: 10.1164/arrd.1978.117.6.1099. [DOI] [PubMed] [Google Scholar]
- Thrall R. S., Barton R. W., D'Amato D. A., Sulavik S. B. Differential cellular analysis of bronchoalveolar lavage fluid obtained at various stages during the development of bleomycin-induced pulmonary fibrosis in the rat. Am Rev Respir Dis. 1982 Sep;126(3):488–492. doi: 10.1164/arrd.1982.126.3.488. [DOI] [PubMed] [Google Scholar]
- Tryka A. F., Godleski J. J., Brain J. D. Alterations in alveolar macrophages in hamsters developing pulmonary fibrosis. Exp Lung Res. 1984;7(1):41–52. doi: 10.3109/01902148409087907. [DOI] [PubMed] [Google Scholar]
- Weinberger S. E., Kelman J. A., Elson N. A., Young R. C., Jr, Reynolds H. Y., Fulmer J. D., Crystal R. G. Bronchoalveolar lavage in interstitial lung disease. Ann Intern Med. 1978 Oct;89(4):459–466. doi: 10.7326/0003-4819-89-4-459. [DOI] [PubMed] [Google Scholar]


