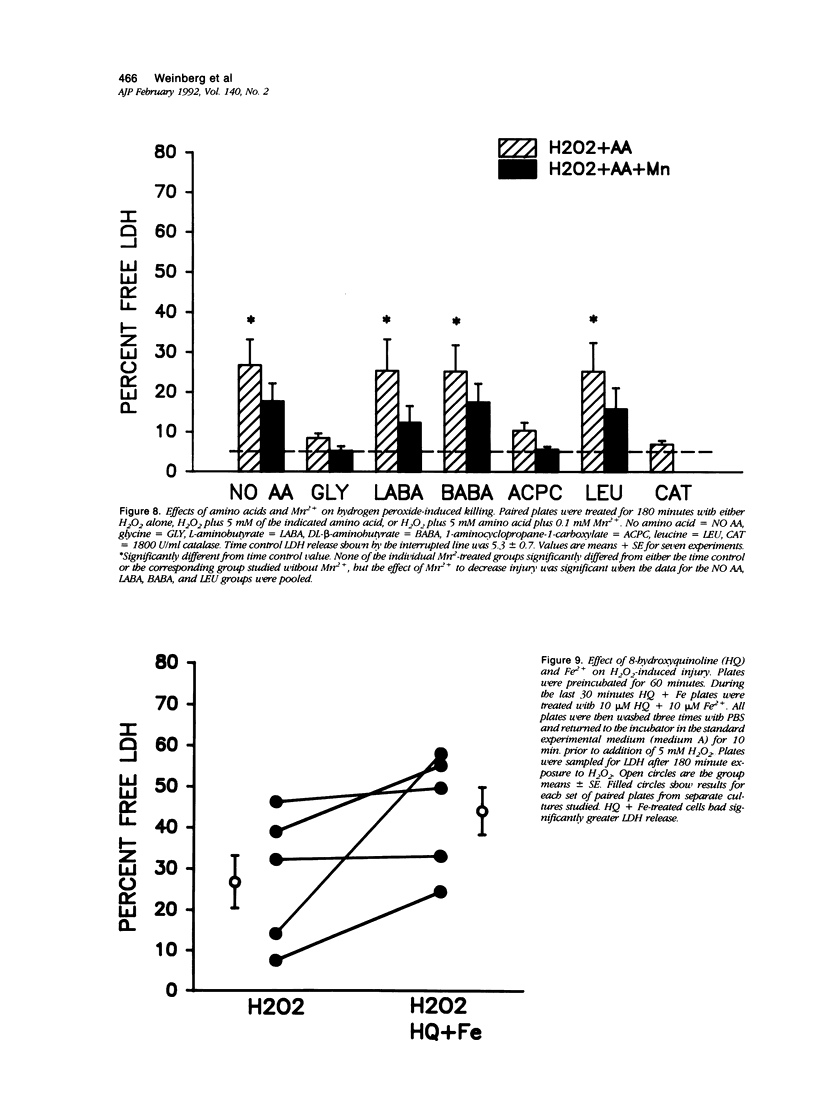
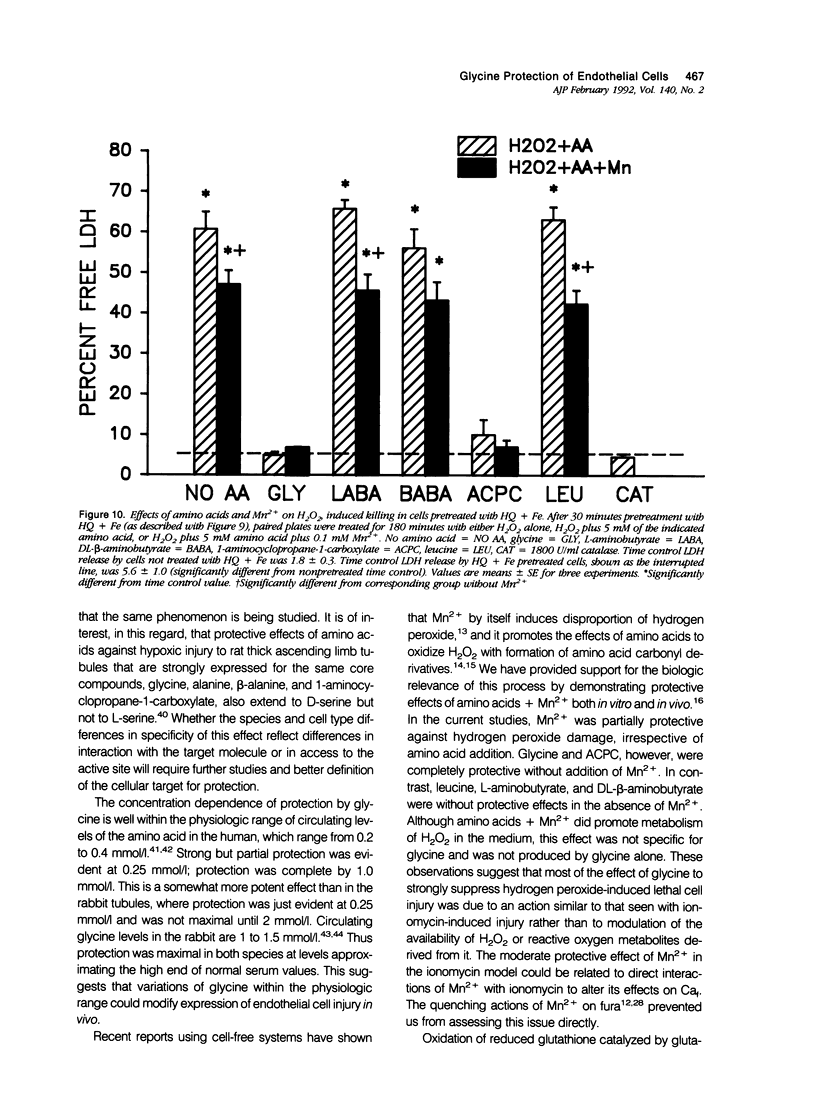
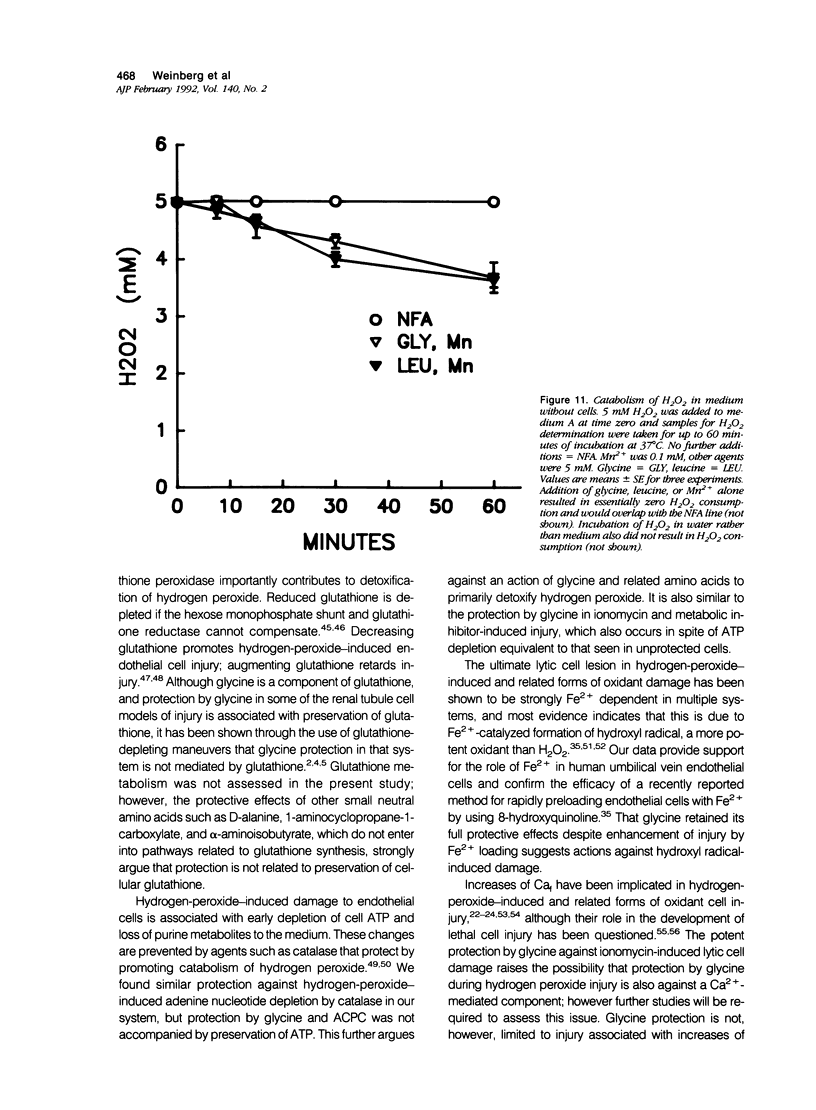
Abstract
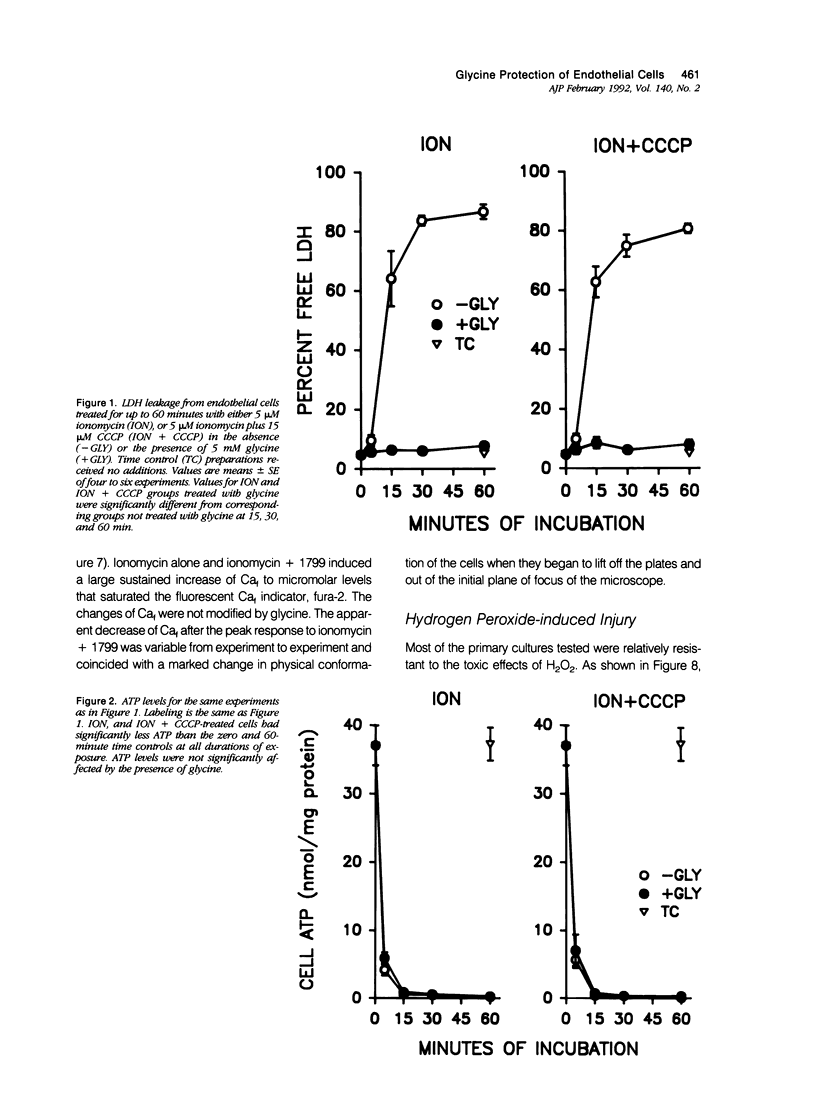
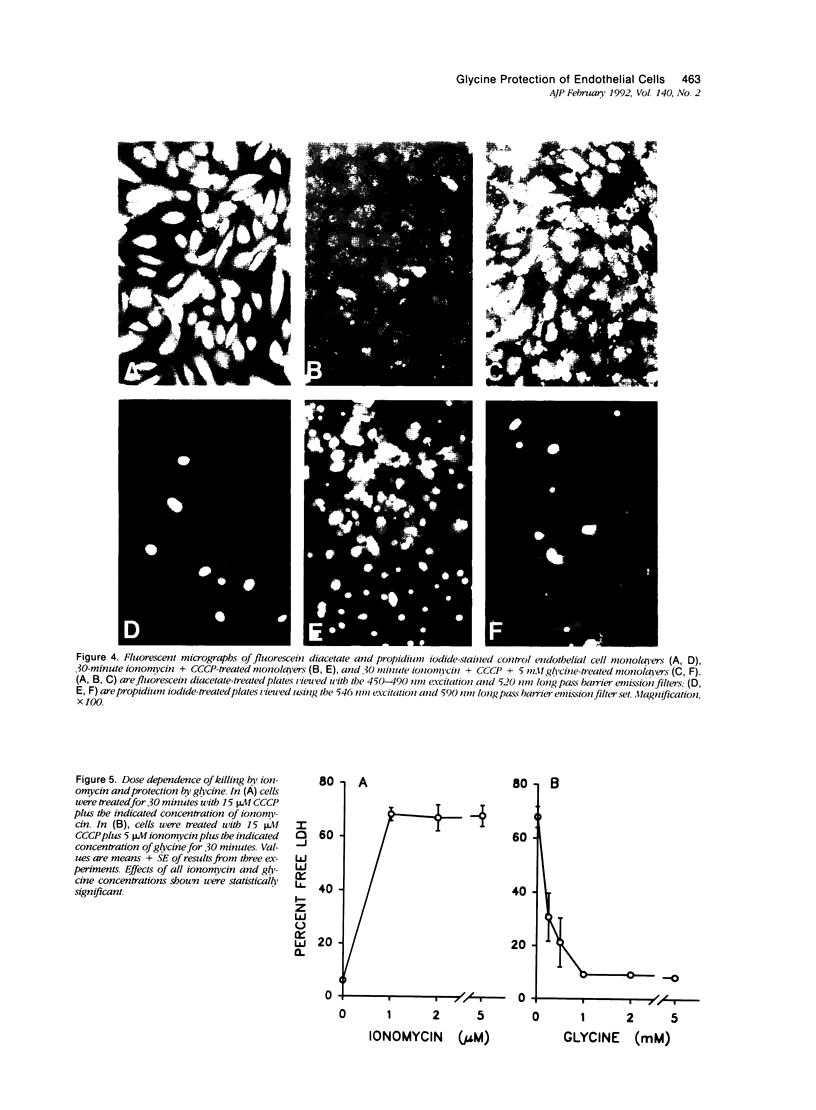
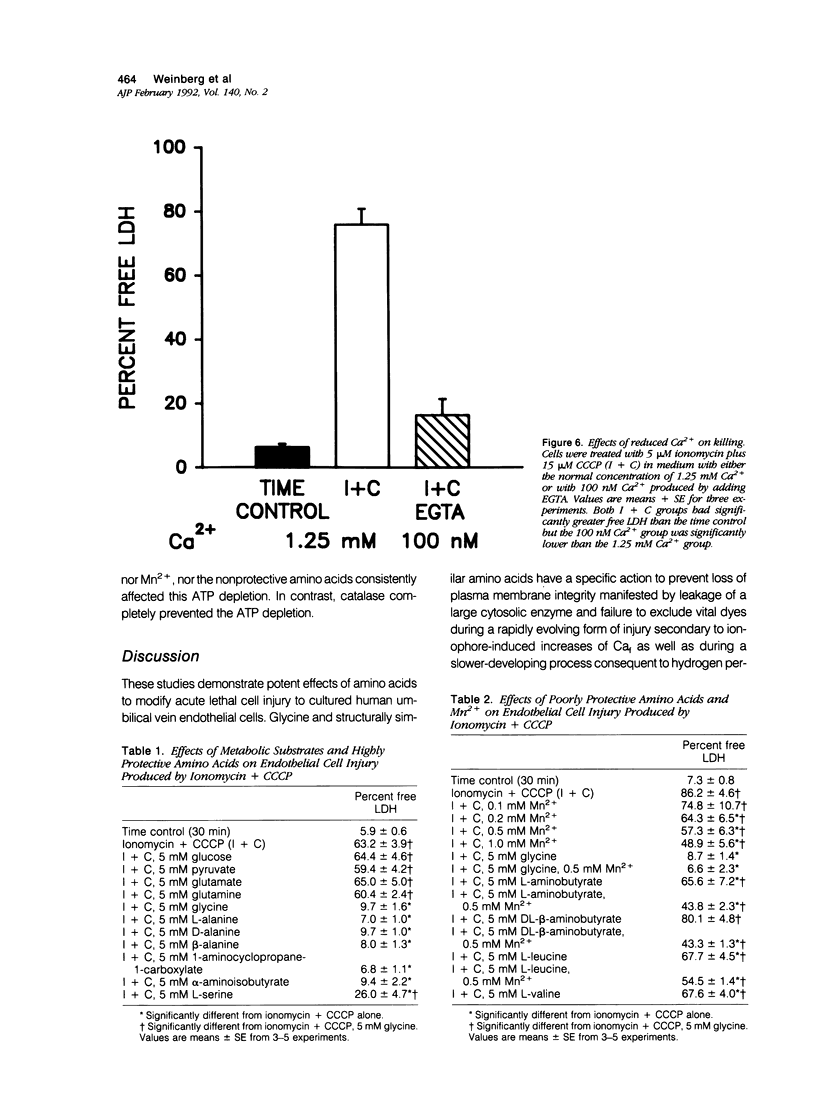
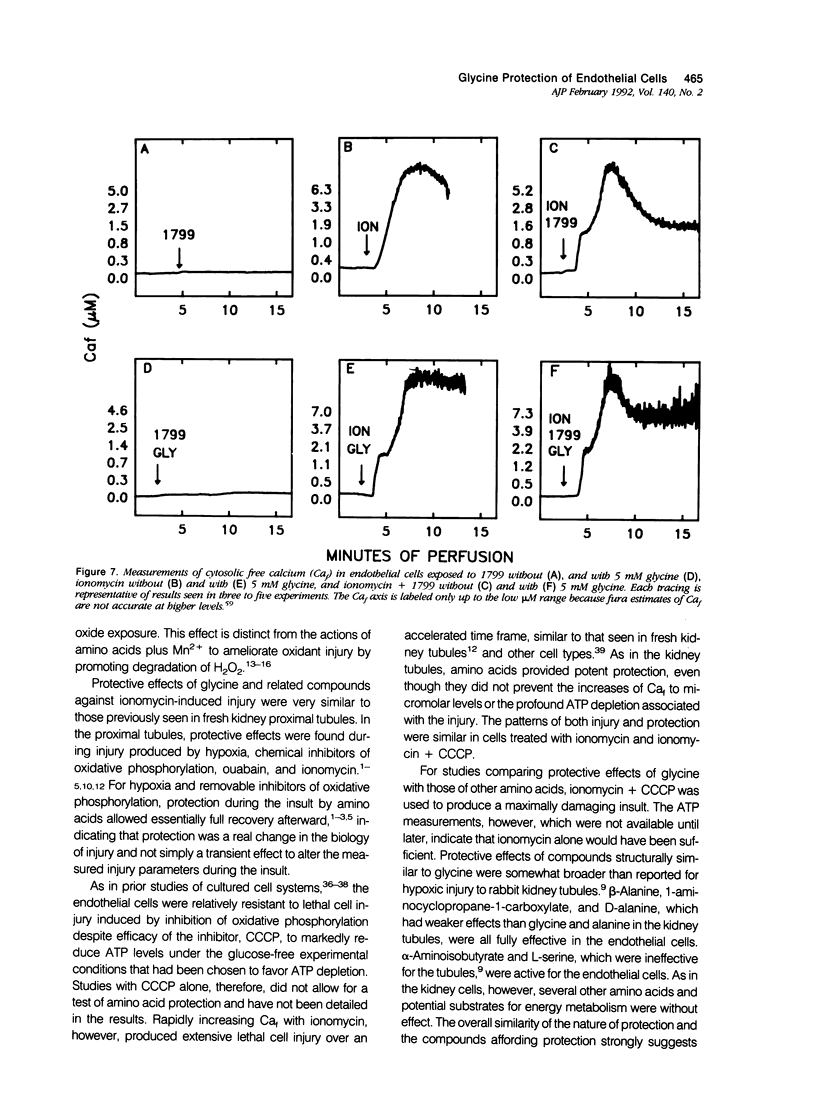
Cultured human umbilical vein endothelial cells treated with either the calcium ionophore, ionomycin, or ionomycin plus cyanide-m-chlorophenylhydrazone had immediate severe depletion of adenosine triphosphate, (ATP) and increases of cytosolic free calcium (Caf) and then sustained lethal cell injury as manifested by release of lactate dehydrogenase and failure to exclude vital dyes within 15 minutes. Inclusion of glycine in the experimental medium prevented the enzyme leakage for at least 60 minutes without altering the ATP depletion or increases of Caf. The physiologic glycine concentration of 0.25 mmol/l gave 50% protection, and protection was complete at 1 mmol/l. Several other small neutral amino acids, L- and D-alanine, beta-alanine, 1-aminocyclopropane-1-carboxylate, alpha-aminoisobutyrate, and L-serine, had effects similar to glycine, but other amino acids and metabolic substrates did not. The endothelial cells were relatively resistant to damage from hydrogen peroxide, but sensitivity could be increased by preloading with Fe2+. In both non-loaded and Fe(2+)-loaded cells, hydrogen-peroxide-induced lactate dehydrogenase (LDH) release developing over 180 minutes was prevented by glycine in a fashion analogous to that seen with ionomycin damage. Mn2+ also partially protected against hydrogen peroxide injury but was not required for glycine's effects. These data demonstrate that striking modulatory effects of glycine and structurally similar amino acids that have previously been characterized in most detail using kidney tubule cells are strongly expressed in human umbilical vein endothelial cells and are involved in their response to Ca2+ and oxidant-mediated damage. These amino acid effects must be considered in the design of in vitro studies of endothelial cell injury and may contribute to endothelial cell pathophysiology in vivo.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ager A., Gordon J. L. Differential effects of hydrogen peroxide on indices of endothelial cell function. J Exp Med. 1984 Feb 1;159(2):592–603. doi: 10.1084/jem.159.2.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreoli S. P., McAteer J. A. Reactive oxygen molecule-mediated injury in endothelial and renal tubular epithelial cells in vitro. Kidney Int. 1990 Nov;38(5):785–794. doi: 10.1038/ki.1990.272. [DOI] [PubMed] [Google Scholar]
- BLOCK W. D., HUBBARD R. W. Amino acid content of rabbit urine and plasma. Arch Biochem Biophys. 1962 Mar;96:557–561. doi: 10.1016/0003-9861(62)90336-3. [DOI] [PubMed] [Google Scholar]
- Baines A. D., Shaikh N., Ho P. Mechanisms of perfused kidney cytoprotection by alanine and glycine. Am J Physiol. 1990 Jul;259(1 Pt 2):F80–F87. doi: 10.1152/ajprenal.1990.259.1.F80. [DOI] [PubMed] [Google Scholar]
- Balla G., Vercellotti G. M., Eaton J. W., Jacob H. S. Iron loading of endothelial cells augments oxidant damage. J Lab Clin Med. 1990 Oct;116(4):546–554. [PubMed] [Google Scholar]
- Berlett B. S., Chock P. B., Yim M. B., Stadtman E. R. Manganese(II) catalyzes the bicarbonate-dependent oxidation of amino acids by hydrogen peroxide and the amino acid-facilitated dismutation of hydrogen peroxide. Proc Natl Acad Sci U S A. 1990 Jan;87(1):389–393. doi: 10.1073/pnas.87.1.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbold P. H., Rink T. J. Fluorescence and bioluminescence measurement of cytoplasmic free calcium. Biochem J. 1987 Dec 1;248(2):313–328. doi: 10.1042/bj2480313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis V. W., Brazy P. C. Sodium, phosphate, glucose, bicarbonate, and alanine interactions in the isolated proximal convoluted tubule of the rabbit kidney. J Clin Invest. 1978 Aug;62(2):387–397. doi: 10.1172/JCI109140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRAME E. G. The levels of individual free amino acids in the plasma of normal man at various intervals after a high-protein meal. J Clin Invest. 1958 Dec;37(12):1710–1723. doi: 10.1172/JCI103763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Computer programs for calculating total from specified free or free from specified total ionic concentrations in aqueous solutions containing multiple metals and ligands. Methods Enzymol. 1988;157:378–417. doi: 10.1016/0076-6879(88)57093-3. [DOI] [PubMed] [Google Scholar]
- Farber J. L., Kyle M. E., Coleman J. B. Mechanisms of cell injury by activated oxygen species. Lab Invest. 1990 Jun;62(6):670–679. [PubMed] [Google Scholar]
- Farber J. L. The role of calcium in lethal cell injury. Chem Res Toxicol. 1990 Nov-Dec;3(6):503–508. doi: 10.1021/tx00018a003. [DOI] [PubMed] [Google Scholar]
- Franceschi D., Graham D., Sarasua M., Zollinger R. M., Jr Mechanisms of oxygen free radical-induced calcium overload in endothelial cells. Surgery. 1990 Aug;108(2):292–297. [PubMed] [Google Scholar]
- Garza-Quintero R., Ortega-Lopez J., Stein J. H., Venkatachalam M. A. Alanine protects rabbit proximal tubules against anoxic injury in vitro. Am J Physiol. 1990 Apr;258(4 Pt 2):F1075–F1083. doi: 10.1152/ajprenal.1990.258.4.F1075. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Halliwell B. Oxidants and human disease: some new concepts. FASEB J. 1987 Nov;1(5):358–364. [PubMed] [Google Scholar]
- Harlan J. M., Levine J. D., Callahan K. S., Schwartz B. R., Harker L. A. Glutathione redox cycle protects cultured endothelial cells against lysis by extracellularly generated hydrogen peroxide. J Clin Invest. 1984 Mar;73(3):706–713. doi: 10.1172/JCI111263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heytler P. G. Uncouplers of oxidative phosphorylation. Methods Enzymol. 1979;55:462–442. doi: 10.1016/0076-6879(79)55060-5. [DOI] [PubMed] [Google Scholar]
- Hinshaw D. B., Armstrong B. C., Beals T. F., Hyslop P. A. A cellular model of endothelial cell ischemia. J Surg Res. 1988 May;44(5):527–537. doi: 10.1016/0022-4804(88)90158-8. [DOI] [PubMed] [Google Scholar]
- Hinshaw D. B., Burger J. M., Armstrong B. C., Hyslop P. A. Mechanism of endothelial cell shape change in oxidant injury. J Surg Res. 1989 Apr;46(4):339–349. doi: 10.1016/0022-4804(89)90199-6. [DOI] [PubMed] [Google Scholar]
- Hyslop P. A., Hinshaw D. B., Schraufstätter I. U., Sklar L. A., Spragg R. G., Cochrane C. G. Intracellular calcium homeostasis during hydrogen peroxide injury to cultured P388D1 cells. J Cell Physiol. 1986 Dec;129(3):356–366. doi: 10.1002/jcp.1041290314. [DOI] [PubMed] [Google Scholar]
- Ikehara T., Yamaguchi H., Hosokawa K., Sakai T., Miyamoto H. Rb+ influx in response to changes in energy generation: effect of the regulation of the ATP content of HeLa cells. J Cell Physiol. 1984 Jun;119(3):273–282. doi: 10.1002/jcp.1041190305. [DOI] [PubMed] [Google Scholar]
- Jewell S. A., Bellomo G., Thor H., Orrenius S., Smith M. Bleb formation in hepatocytes during drug metabolism is caused by disturbances in thiol and calcium ion homeostasis. Science. 1982 Sep 24;217(4566):1257–1259. doi: 10.1126/science.7112127. [DOI] [PubMed] [Google Scholar]
- Jones K. H., Senft J. A. An improved method to determine cell viability by simultaneous staining with fluorescein diacetate-propidium iodide. J Histochem Cytochem. 1985 Jan;33(1):77–79. doi: 10.1177/33.1.2578146. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mandel L. J., Schnellmann R. G., Jacobs W. R. Intracellular glutathione in the protection from anoxic injury in renal proximal tubules. J Clin Invest. 1990 Feb;85(2):316–324. doi: 10.1172/JCI114440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt J. E., Jacob R., Hallam T. J. Use of manganese to discriminate between calcium influx and mobilization from internal stores in stimulated human neutrophils. J Biol Chem. 1989 Jan 25;264(3):1522–1527. [PubMed] [Google Scholar]
- Phelps P. C., Smith M. W., Trump B. F. Cytosolic ionized calcium and bleb formation after acute cell injury of cultured rabbit renal tubule cells. Lab Invest. 1989 May;60(5):630–642. [PubMed] [Google Scholar]
- Sacks T., Moldow C. F., Craddock P. R., Bowers T. K., Jacob H. S. Oxygen radicals mediate endothelial cell damage by complement-stimulated granulocytes. An in vitro model of immune vascular damage. J Clin Invest. 1978 May;61(5):1161–1167. doi: 10.1172/JCI109031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schanne F. A., Kane A. B., Young E. E., Farber J. L. Calcium dependence of toxic cell death: a final common pathway. Science. 1979 Nov 9;206(4419):700–702. doi: 10.1126/science.386513. [DOI] [PubMed] [Google Scholar]
- Schuger L., Varani J., Marks R. M., Kunkel S. L., Johnson K. J., Ward P. A. Cytotoxicity of tumor necrosis factor-alpha for human umbilical vein endothelial cells. Lab Invest. 1989 Jul;61(1):62–68. [PubMed] [Google Scholar]
- Shasby D. M., Lind S. E., Shasby S. S., Goldsmith J. C., Hunninghake G. W. Reversible oxidant-induced increases in albumin transfer across cultured endothelium: alterations in cell shape and calcium homeostasis. Blood. 1985 Mar;65(3):605–614. [PubMed] [Google Scholar]
- Shasby D. M., Yorek M., Shasby S. S. Exogenous oxidants initiate hydrolysis of endothelial cell inositol phospholipids. Blood. 1988 Aug;72(2):491–499. [PubMed] [Google Scholar]
- Stadtman E. R., Berlett B. S., Chock P. B. Manganese-dependent disproportionation of hydrogen peroxide in bicarbonate buffer. Proc Natl Acad Sci U S A. 1990 Jan;87(1):384–388. doi: 10.1073/pnas.87.1.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starke P. E., Hoek J. B., Farber J. L. Calcium-dependent and calcium-independent mechanisms of irreversible cell injury in cultured hepatocytes. J Biol Chem. 1986 Mar 5;261(7):3006–3012. [PubMed] [Google Scholar]
- Steinberg S. F., Bilezikian J. P., Al-Awqati Q. Fura-2 fluorescence is localized to mitochondria in endothelial cells. Am J Physiol. 1987 Nov;253(5 Pt 1):C744–C747. doi: 10.1152/ajpcell.1987.253.5.C744. [DOI] [PubMed] [Google Scholar]
- Suttorp N., Toepfer W., Roka L. Antioxidant defense mechanisms of endothelial cells: glutathione redox cycle versus catalase. Am J Physiol. 1986 Nov;251(5 Pt 1):C671–C680. doi: 10.1152/ajpcell.1986.251.5.C671. [DOI] [PubMed] [Google Scholar]
- Tsan M. F., White J. E., Rosano C. L. Modulation of endothelial GSH concentrations: effect of exogenous GSH and GSH monoethyl ester. J Appl Physiol (1985) 1989 Mar;66(3):1029–1034. doi: 10.1152/jappl.1989.66.3.1029. [DOI] [PubMed] [Google Scholar]
- Varani J., Ginsburg I., Gibbs D. F., Mukhopadhyay P. S., Sulavik C., Johnson K. J., Weinberg J. M., Ryan U. S., Ward P. A. Hydrogen peroxide-induced cell and tissue injury: protective effects of Mn2+. Inflammation. 1991 Aug;15(4):291–301. doi: 10.1007/BF00917314. [DOI] [PubMed] [Google Scholar]
- Venkatachalam M. A., Patel Y. J., Kreisberg J. I., Weinberg J. M. Energy thresholds that determine membrane integrity and injury in a renal epithelial cell line (LLC-PK1). Relationships to phospholipid degradation and unesterified fatty acid accumulation. J Clin Invest. 1988 Mar;81(3):745–758. doi: 10.1172/JCI113380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercellotti G. M., Severson S. P., Duane P., Moldow C. F. Hydrogen peroxide alters signal transduction in human endothelial cells. J Lab Clin Med. 1991 Jan;117(1):15–24. [PubMed] [Google Scholar]
- Weinberg J. M., Buchanan D. N., Davis J. A., Abarzua M. Metabolic aspects of protection by glycine against hypoxic injury to isolated proximal tubules. J Am Soc Nephrol. 1991 Jan;1(7):949–958. doi: 10.1681/ASN.V17949. [DOI] [PubMed] [Google Scholar]
- Weinberg J. M., Davis J. A., Abarzua M., Kiani T. Relationship between cell adenosine triphosphate and glutathione content and protection by glycine against hypoxic proximal tubule cell injury. J Lab Clin Med. 1989 May;113(5):612–622. [PubMed] [Google Scholar]
- Weinberg J. M., Davis J. A., Abarzua M., Rajan T. Cytoprotective effects of glycine and glutathione against hypoxic injury to renal tubules. J Clin Invest. 1987 Nov;80(5):1446–1454. doi: 10.1172/JCI113224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg J. M., Davis J. A., Abarzua M., Smith R. K., Kunkel R. Ouabain-induced lethal proximal tubule cell injury is prevented by glycine. Am J Physiol. 1990 Feb;258(2 Pt 2):F346–F355. doi: 10.1152/ajprenal.1990.258.2.F346. [DOI] [PubMed] [Google Scholar]
- Weinberg J. M., Venkatachalam M. A., Garzo-Quintero R., Roeser N. F., Davis J. A. Structural requirements for protection by small amino acids against hypoxic injury in kidney proximal tubules. FASEB J. 1990 Dec;4(15):3347–3354. doi: 10.1096/fasebj.4.15.2253849. [DOI] [PubMed] [Google Scholar]
- Weiss S. J., Young J., LoBuglio A. F., Slivka A., Nimeh N. F. Role of hydrogen peroxide in neutrophil-mediated destruction of cultured endothelial cells. J Clin Invest. 1981 Sep;68(3):714–721. doi: 10.1172/JCI110307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim M. B., Berlett B. S., Chock P. B., Stadtman E. R. Manganese(II)-bicarbonate-mediated catalytic activity for hydrogen peroxide dismutation and amino acid oxidation: detection of free radical intermediates. Proc Natl Acad Sci U S A. 1990 Jan;87(1):394–398. doi: 10.1073/pnas.87.1.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. A., Freedman B. S. Renal tubular transport of amino acids. Clin Chem. 1971 Apr;17(4):245–266. [PubMed] [Google Scholar]
- Zweier J. L., Kuppusamy P., Lutty G. A. Measurement of endothelial cell free radical generation: evidence for a central mechanism of free radical injury in postischemic tissues. Proc Natl Acad Sci U S A. 1988 Jun;85(11):4046–4050. doi: 10.1073/pnas.85.11.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]




