Abstract
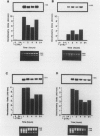
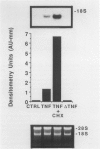
Chemotactic cytokines play a critical role in recruiting leukocytes to sites of tissue injury. Interleukin-8 (IL-8) is a chemotactic cytokine secreted by a variety of cells (eg, monocytes, endothelial cells, fibroblasts) during the inflammatory response. In this report, the authors demonstrate that human transitional cell carcinomas and renal cell carcinomas have the capacity to elaborate IL-8 in response to the inflammatory mediators IL-1 beta and tumor necrosis factor (TNF)-alpha. All cell lines expressed high levels of IL-8 mRNA on stimulation with either IL-1 beta or TNF-alpha, but not lipopolysaccharide; one expressed the gene constitutively. The authors selected one transitional cell carcinoma cell line (UM-UC-9) and one renal cell carcinoma cell line (UM-RC-5) for further study. Both displayed a time- and dose-dependent increase in steady-state levels of IL-8 mRNA in response to IL-1 beta and TNF-alpha. Specific mRNA was detectable by 1 hour after stimulation. Secretion of antigenic IL-8 measured by enzyme-linked immunosorbent assay into culture supernatants reflected the kinetics of mRNA expression. Because heat-inactivated TNF-alpha failed to induce synthesis of IL-8 mRNA, and cycloheximide augmented TNF-alpha-induced synthesis, IL-8 expression appears to be a stimulus-specific primary induction phenomenon. As with other inflammatory mediators whose mRNA contains a 3' AU-rich sequence (eg, IL-2, TNF-alpha), the half-life of IL-8 mRNA was short, less than 1 hour. Our data suggest that secretion of IL-8 by malignant cells may partly account for the inflammatory infiltrates associated with some malignant neoplasms.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caput D., Beutler B., Hartog K., Thayer R., Brown-Shimer S., Cerami A. Identification of a common nucleotide sequence in the 3'-untranslated region of mRNA molecules specifying inflammatory mediators. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1670–1674. doi: 10.1073/pnas.83.6.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Collart M. A., Belin D., Vassalli J. D., de Kossodo S., Vassalli P. Gamma interferon enhances macrophage transcription of the tumor necrosis factor/cachectin, interleukin 1, and urokinase genes, which are controlled by short-lived repressors. J Exp Med. 1986 Dec 1;164(6):2113–2118. doi: 10.1084/jem.164.6.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeForge L. E., Remick D. G. Kinetics of TNF, IL-6, and IL-8 gene expression in LPS-stimulated human whole blood. Biochem Biophys Res Commun. 1991 Jan 15;174(1):18–24. doi: 10.1016/0006-291x(91)90478-p. [DOI] [PubMed] [Google Scholar]
- Graves D. T., Jiang Y. L., Williamson M. J., Valente A. J. Identification of monocyte chemotactic activity produced by malignant cells. Science. 1989 Sep 29;245(4925):1490–1493. doi: 10.1126/science.2781291. [DOI] [PubMed] [Google Scholar]
- Grossman H. B., Wedemeyer G., Ren L. Q. Human renal carcinoma: characterization of five new cell lines. J Surg Oncol. 1985 Mar;28(3):237–244. doi: 10.1002/jso.2930280320. [DOI] [PubMed] [Google Scholar]
- Grossman H. B., Wedemeyer G., Ren L., Wilson G. N., Cox B. Improved growth of human urothelial carcinoma cell cultures. J Urol. 1986 Oct;136(4):953–959. doi: 10.1016/s0022-5347(17)45139-1. [DOI] [PubMed] [Google Scholar]
- Jonas E., Sargent T. D., Dawid I. B. Epidermal keratin gene expressed in embryos of Xenopus laevis. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5413–5417. doi: 10.1073/pnas.82.16.5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen C. G., Anderson A. O., Appella E., Oppenheim J. J., Matsushima K. The neutrophil-activating protein (NAP-1) is also chemotactic for T lymphocytes. Science. 1989 Mar 17;243(4897):1464–1466. doi: 10.1126/science.2648569. [DOI] [PubMed] [Google Scholar]
- Larsen C. G., Anderson A. O., Oppenheim J. J., Matsushima K. Production of interleukin-8 by human dermal fibroblasts and keratinocytes in response to interleukin-1 or tumour necrosis factor. Immunology. 1989 Sep;68(1):31–36. [PMC free article] [PubMed] [Google Scholar]
- Liebert M., Wedemeyer G., Abruzzo L. V., Kunkel S. L., Hammerberg C., Cooper K. D., Grossman H. B. Stimulated urothelial cells produce cytokines and express an activated cell surface antigenic phenotype. Semin Urol. 1991 May;9(2):124–130. [PubMed] [Google Scholar]
- Malter J. S. Identification of an AUUUA-specific messenger RNA binding protein. Science. 1989 Nov 3;246(4930):664–666. doi: 10.1126/science.2814487. [DOI] [PubMed] [Google Scholar]
- Mancilla-Jimenez R., Stanley R. J., Blath R. A. Papillary renal cell carcinoma: a clinical, radiologic, and pathologic study of 34 cases. Cancer. 1976 Dec;38(6):2469–2480. doi: 10.1002/1097-0142(197612)38:6<2469::aid-cncr2820380636>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Matsushima K., Morishita K., Yoshimura T., Lavu S., Kobayashi Y., Lew W., Appella E., Kung H. F., Leonard E. J., Oppenheim J. J. Molecular cloning of a human monocyte-derived neutrophil chemotactic factor (MDNCF) and the induction of MDNCF mRNA by interleukin 1 and tumor necrosis factor. J Exp Med. 1988 Jun 1;167(6):1883–1893. doi: 10.1084/jem.167.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peveri P., Walz A., Dewald B., Baggiolini M. A novel neutrophil-activating factor produced by human mononuclear phagocytes. J Exp Med. 1988 May 1;167(5):1547–1559. doi: 10.1084/jem.167.5.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma K. P. The role of lymphoid reaction in bladder cancer. J Urol. 1970 Dec;104(6):843–849. doi: 10.1016/s0022-5347(17)61849-4. [DOI] [PubMed] [Google Scholar]
- Schröder J. M., Christophers E. Identification of C5ades arg and an anionic neutrophil-activating peptide (ANAP) in psoriatic scales. J Invest Dermatol. 1986 Jul;87(1):53–58. doi: 10.1111/1523-1747.ep12523566. [DOI] [PubMed] [Google Scholar]
- Schröder J. M., Mrowietz U., Morita E., Christophers E. Purification and partial biochemical characterization of a human monocyte-derived, neutrophil-activating peptide that lacks interleukin 1 activity. J Immunol. 1987 Nov 15;139(10):3474–3483. [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Standiford T. J., Strieter R. M., Chensue S. W., Westwick J., Kasahara K., Kunkel S. L. IL-4 inhibits the expression of IL-8 from stimulated human monocytes. J Immunol. 1990 Sep 1;145(5):1435–1439. [PubMed] [Google Scholar]
- Strieter R. M., Kunkel S. L., Showell H. J., Remick D. G., Phan S. H., Ward P. A., Marks R. M. Endothelial cell gene expression of a neutrophil chemotactic factor by TNF-alpha, LPS, and IL-1 beta. Science. 1989 Mar 17;243(4897):1467–1469. doi: 10.1126/science.2648570. [DOI] [PubMed] [Google Scholar]
- Strieter R. M., Phan S. H., Showell H. J., Remick D. G., Lynch J. P., Genord M., Raiford C., Eskandari M., Marks R. M., Kunkel S. L. Monokine-induced neutrophil chemotactic factor gene expression in human fibroblasts. J Biol Chem. 1989 Jun 25;264(18):10621–10626. [PubMed] [Google Scholar]
- Thelen M., Peveri P., Kernen P., von Tscharner V., Walz A., Baggiolini M. Mechanism of neutrophil activation by NAF, a novel monocyte-derived peptide agonist. FASEB J. 1988 Aug;2(11):2702–2706. [PubMed] [Google Scholar]
- Thornton A. J., Strieter R. M., Lindley I., Baggiolini M., Kunkel S. L. Cytokine-induced gene expression of a neutrophil chemotactic factor/IL-8 in human hepatocytes. J Immunol. 1990 Apr 1;144(7):2609–2613. [PubMed] [Google Scholar]
- Van Damme J., Decock B., Conings R., Lenaerts J. P., Opdenakker G., Billiau A. The chemotactic activity for granulocytes produced by virally infected fibroblasts is identical to monocyte-derived interleukin 8. Eur J Immunol. 1989 Jul;19(7):1189–1194. doi: 10.1002/eji.1830190706. [DOI] [PubMed] [Google Scholar]
- Walz A., Peveri P., Aschauer H., Baggiolini M. Purification and amino acid sequencing of NAF, a novel neutrophil-activating factor produced by monocytes. Biochem Biophys Res Commun. 1987 Dec 16;149(2):755–761. doi: 10.1016/0006-291x(87)90432-3. [DOI] [PubMed] [Google Scholar]
- Yoshimura T., Matsushima K., Tanaka S., Robinson E. A., Appella E., Oppenheim J. J., Leonard E. J. Purification of a human monocyte-derived neutrophil chemotactic factor that has peptide sequence similarity to other host defense cytokines. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9233–9237. doi: 10.1073/pnas.84.24.9233. [DOI] [PMC free article] [PubMed] [Google Scholar]