Abstract
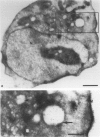
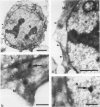
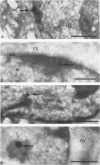
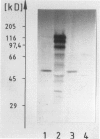
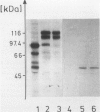
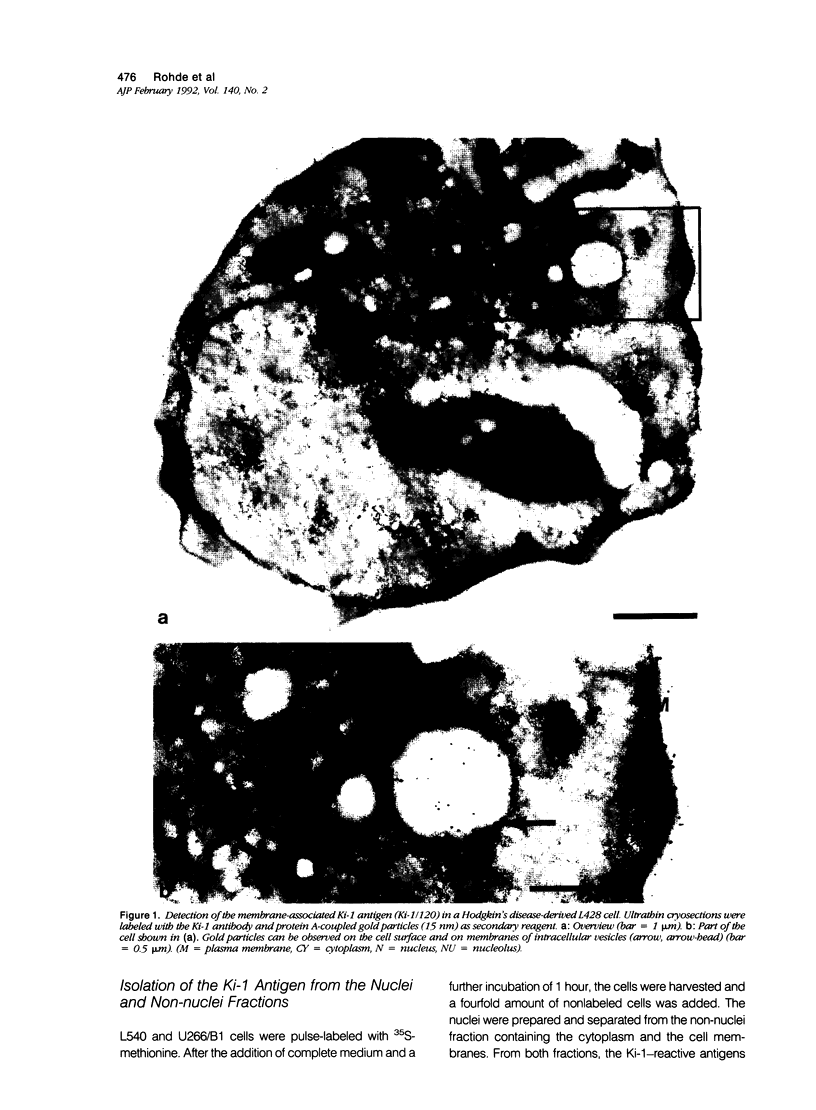
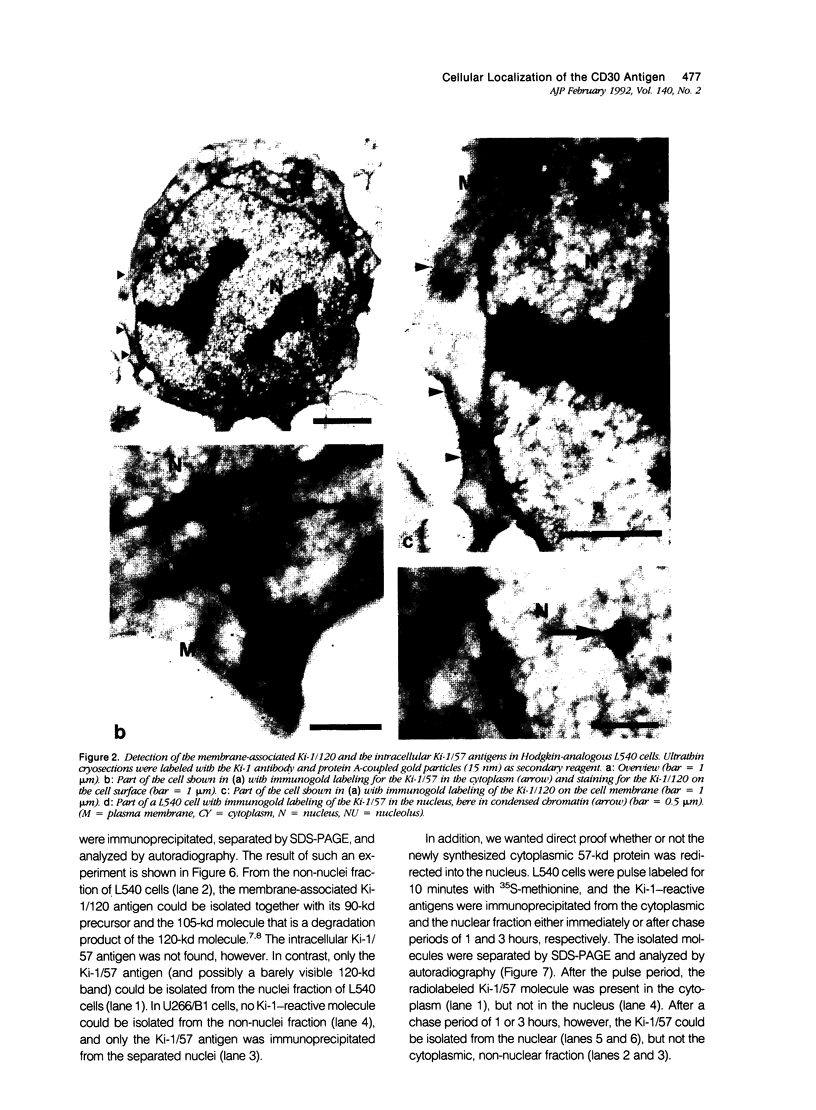
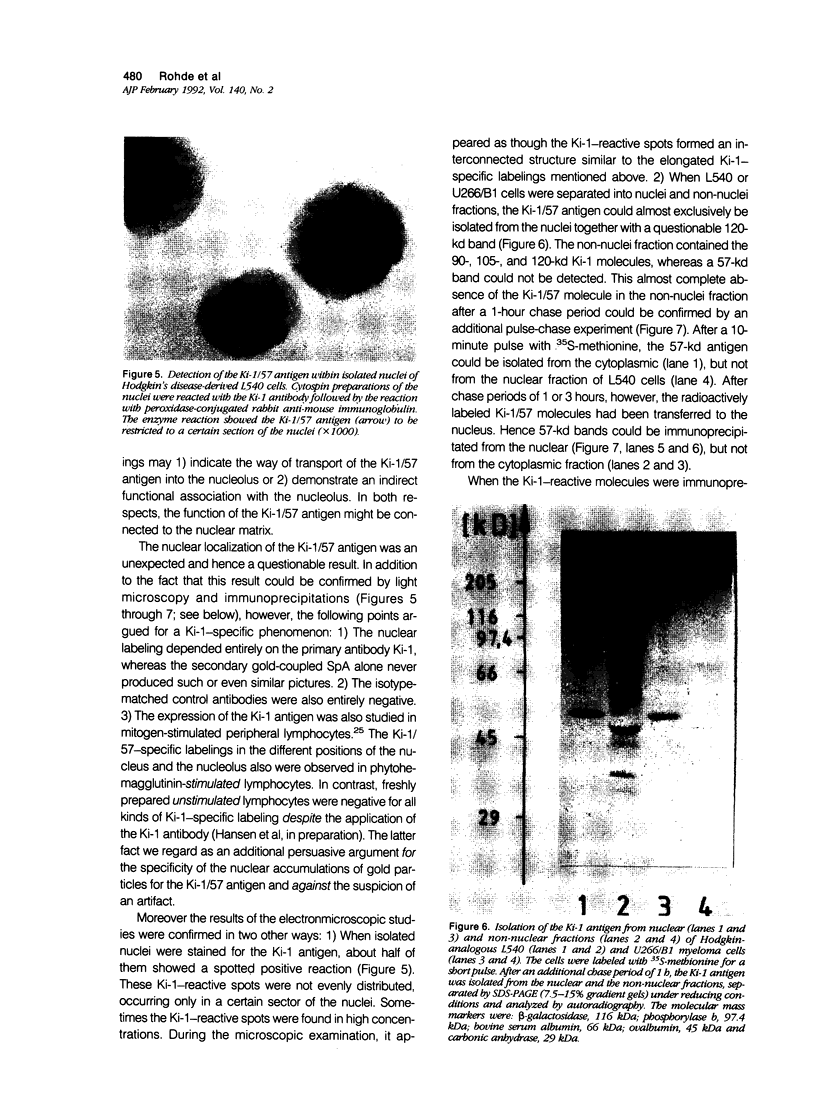
The Ki-1 antibody not only detects a Hodgkin-associated membrane molecule of 120 kd (Ki-1/120 = CD30), but also reacts with an independently synthesized molecule of 57 kd (Ki-1/57) that only occurs intracellularly. Hodgkin's disease-derived cell lines L428 and L540 contain both Ki-1-reactive antigens, whereas others, e.g., U266/Bl myeloma cells, only express the intracellular Ki-1/57. The present immunoelectronmicroscopic analysis detected the Ki-1/57 antigen of U266/Bl cells not only in the cytoplasm, but also in association with the nuclear envelope, chromatin structures, and nucleoli. This Ki-1/57-specific type of labeling also was observed in L428 and L540 cells that, in contrast to U266/Bl cells, showed an additional staining of cell membranes and cytoplasmic vesicles. These results were confirmed by two independent methods: 1) cytocentrifuge preparations of isolated nuclei of L540 cells showed a spotted Ki-1-specific labeling, 2) immunoprecipitations demonstrated that the Ki-1/57, but not the Ki-1/120 antigen, was transferred into the nuclei of L540 and U266/Bl cells, whereas the Ki-1/120 antigen with its 90-kd precursor remained in the non-nuclei fraction of L540 cells.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreesen R., Brugger W., Löhr G. W., Bross K. J. Human macrophages can express the Hodgkin's cell-associated antigen Ki-1 (CD30). Am J Pathol. 1989 Jan;134(1):187–192. [PMC free article] [PubMed] [Google Scholar]
- Andreesen R., Osterholz J., Löhr G. W., Bross K. J. A Hodgkin cell-specific antigen is expressed on a subset of auto- and alloactivated T (helper) lymphoblasts. Blood. 1984 Jun;63(6):1299–1302. [PubMed] [Google Scholar]
- Bergmann J. E., Singer S. J. Immunoelectron microscopic studies of the intracellular transport of the membrane glycoprotein (G) of vesicular stomatitis virus in infected Chinese hamster ovary cells. J Cell Biol. 1983 Dec;97(6):1777–1787. doi: 10.1083/jcb.97.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerr-Schott J., Garaud J. C. Ultrastructural identification of Gastrin-like immunoreactive nerve fibres in the brain of Xenopus laevis by means of colloidal gold or ferritin immunocytochemical methods. Cell Tissue Res. 1981;216(3):581–589. doi: 10.1007/BF00238653. [DOI] [PubMed] [Google Scholar]
- Eldred W. D., Zucker C., Karten H. J., Yazulla S. Comparison of fixation and penetration enhancement techniques for use in ultrastructural immunocytochemistry. J Histochem Cytochem. 1983 Feb;31(2):285–292. doi: 10.1177/31.2.6339606. [DOI] [PubMed] [Google Scholar]
- Froese P., Lemke H., Gerdes J., Havsteen B., Schwarting R., Hansen H., Stein H. Biochemical characterization and biosynthesis of the Ki-1 antigen in Hodgkin-derived and virus-transformed human B and T lymphoid cell lines. J Immunol. 1987 Sep 15;139(6):2081–2087. [PubMed] [Google Scholar]
- Hansen H., Bredfeldt G., Havsteen B., Lemke H. Protein kinase activity of the intracellular but not of the membrane-associated form of the Ki-1 antigen (CD30). Res Immunol. 1990 Jan;141(1):13–31. doi: 10.1016/0923-2494(90)90098-j. [DOI] [PubMed] [Google Scholar]
- Hansen H., Lemke H., Bredfeldt G., Könnecke I., Havsteen B. The Hodgkin-associated Ki-1 antigen exists in an intracellular and a membrane-bound form. Biol Chem Hoppe Seyler. 1989 May;370(5):409–416. doi: 10.1515/bchm3.1989.370.1.409. [DOI] [PubMed] [Google Scholar]
- Hearn S. A., Silver M. M., Sholdice J. A. Immunoelectron microscopic labeling of immunoglobulin in plasma cells after osmium fixation and epoxy embedding. J Histochem Cytochem. 1985 Dec;33(12):1212–1218. doi: 10.1177/33.12.3934258. [DOI] [PubMed] [Google Scholar]
- Jones D. B. The histogenesis of the Reed-Sternberg cell and its mononuclear counterparts. J Pathol. 1987 Mar;151(3):191–195. doi: 10.1002/path.1711510306. [DOI] [PubMed] [Google Scholar]
- Josimovic-Alasevic O., Dürkop H., Schwarting R., Backé E., Stein H., Diamantstein T. Ki-1 (CD30) antigen is released by Ki-1-positive tumor cells in vitro and in vivo. I. Partial characterization of soluble Ki-1 antigen and detection of the antigen in cell culture supernatants and in serum by an enzyme-linked immunosorbent assay. Eur J Immunol. 1989 Jan;19(1):157–162. doi: 10.1002/eji.1830190125. [DOI] [PubMed] [Google Scholar]
- Kamesaki H., Fukuhara S., Tatsumi E., Uchino H., Yamabe H., Miwa H., Shirakawa S., Hatanaka M., Honjo T. Cytochemical, immunologic, chromosomal, and molecular genetic analysis of a novel cell line derived from Hodgkin's disease. Blood. 1986 Jul;68(1):285–292. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lemke H., Hämmerling G. J., Hämmerling U. Fine specificity analysis with monoclonal antibodies of antigens controlled by the major histocompatibility complex and by the Qa/TL region in mice. Immunol Rev. 1979;47:175–206. doi: 10.1111/j.1600-065x.1979.tb00293.x. [DOI] [PubMed] [Google Scholar]
- Lemke H., Krausse R., Lorenzen J., Havsteen B. Mycoplasma infection of cell lines can simulate the expression of Fc receptors by binding of the carbohydrate moiety of antibodies. Eur J Immunol. 1985 May;15(5):442–447. doi: 10.1002/eji.1830150506. [DOI] [PubMed] [Google Scholar]
- Nawrocki J. F., Kirsten E. S., Fisher R. I. Biochemical and structural properties of a Hodgkin's disease-related membrane protein. J Immunol. 1988 Jul 15;141(2):672–680. [PubMed] [Google Scholar]
- Nilsson K., Bennich H., Johansson S. G., Pontén J. Established immunoglobulin producing myeloma (IgE) and lymphoblastoid (IgG) cell lines from an IgE myeloma patient. Clin Exp Immunol. 1970 Oct;7(4):477–489. [PMC free article] [PubMed] [Google Scholar]
- Pizzolo G., Vinante F., Chilosi M., Dallenbach F., Josimovic-Alasevic O., Diamantstein T., Stein H. Serum levels of soluble CD30 molecule (Ki-1 antigen) in Hodgkin's disease: relationship with disease activity and clinical stage. Br J Haematol. 1990 Jun;75(2):282–284. doi: 10.1111/j.1365-2141.1990.tb02664.x. [DOI] [PubMed] [Google Scholar]
- Roth J. Post-embedding cytochemistry with gold-labelled reagents: a review. J Microsc. 1986 Aug;143(Pt 2):125–137. doi: 10.1111/j.1365-2818.1986.tb02771.x. [DOI] [PubMed] [Google Scholar]
- Schaadt M., Burrichter H., Stein H., Pfreundschuh M., Fonatsch C., Diehl V. The cell of origin in Hodgkin's disease: conclusions from in vivo and in vitro studies. Int Rev Exp Pathol. 1985;27:185–202. [PubMed] [Google Scholar]
- Schwab U., Stein H., Gerdes J., Lemke H., Kirchner H., Schaadt M., Diehl V. Production of a monoclonal antibody specific for Hodgkin and Sternberg-Reed cells of Hodgkin's disease and a subset of normal lymphoid cells. Nature. 1982 Sep 2;299(5878):65–67. doi: 10.1038/299065a0. [DOI] [PubMed] [Google Scholar]
- Stein H., Gerdes J., Lemke H., Mason D. Y. Evidence of Sternberg-Reed cells being derived from activated lymphocytes. Haematol Blood Transfus. 1985;29:441–444. doi: 10.1007/978-3-642-70385-0_91. [DOI] [PubMed] [Google Scholar]
- Stein H., Gerdes J., Schwab U., Lemke H., Mason D. Y., Ziegler A., Schienle W., Diehl V. Identification of Hodgkin and Sternberg-reed cells as a unique cell type derived from a newly-detected small-cell population. Int J Cancer. 1982 Oct 15;30(4):445–459. doi: 10.1002/ijc.2910300411. [DOI] [PubMed] [Google Scholar]
- Stein H., Mason D. Y., Gerdes J., O'Connor N., Wainscoat J., Pallesen G., Gatter K., Falini B., Delsol G., Lemke H. The expression of the Hodgkin's disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood. 1985 Oct;66(4):848–858. [PubMed] [Google Scholar]
- Steinman R. M., Mellman I. S., Muller W. A., Cohn Z. A. Endocytosis and the recycling of plasma membrane. J Cell Biol. 1983 Jan;96(1):1–27. doi: 10.1083/jcb.96.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyasu K. T. Immunochemistry on ultrathin frozen sections. Histochem J. 1980 Jul;12(4):381–403. doi: 10.1007/BF01011956. [DOI] [PubMed] [Google Scholar]