Abstract
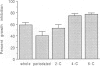
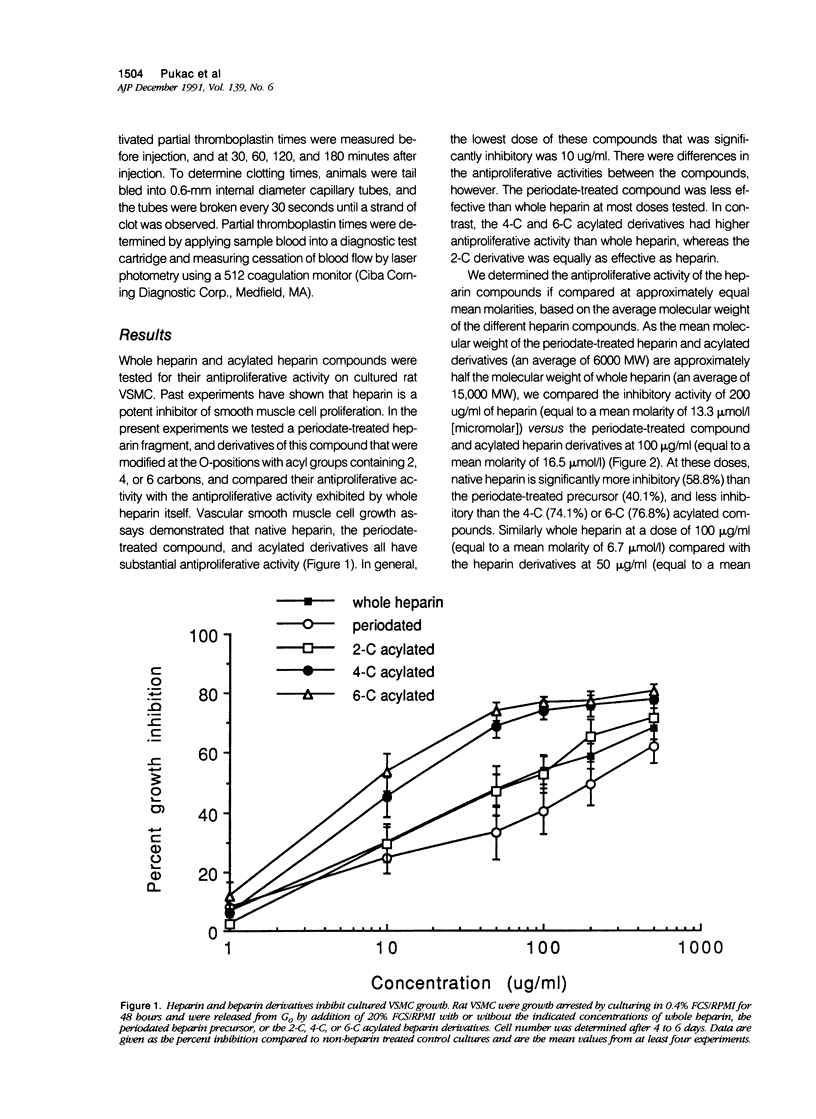
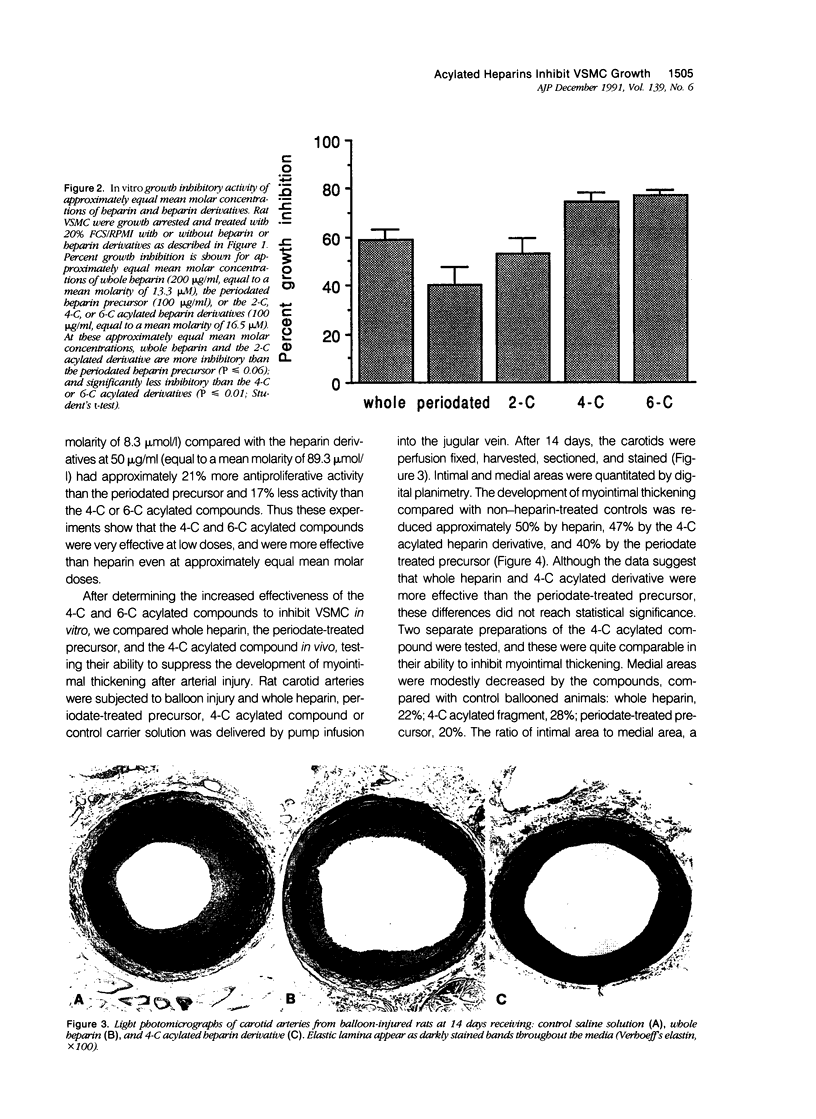
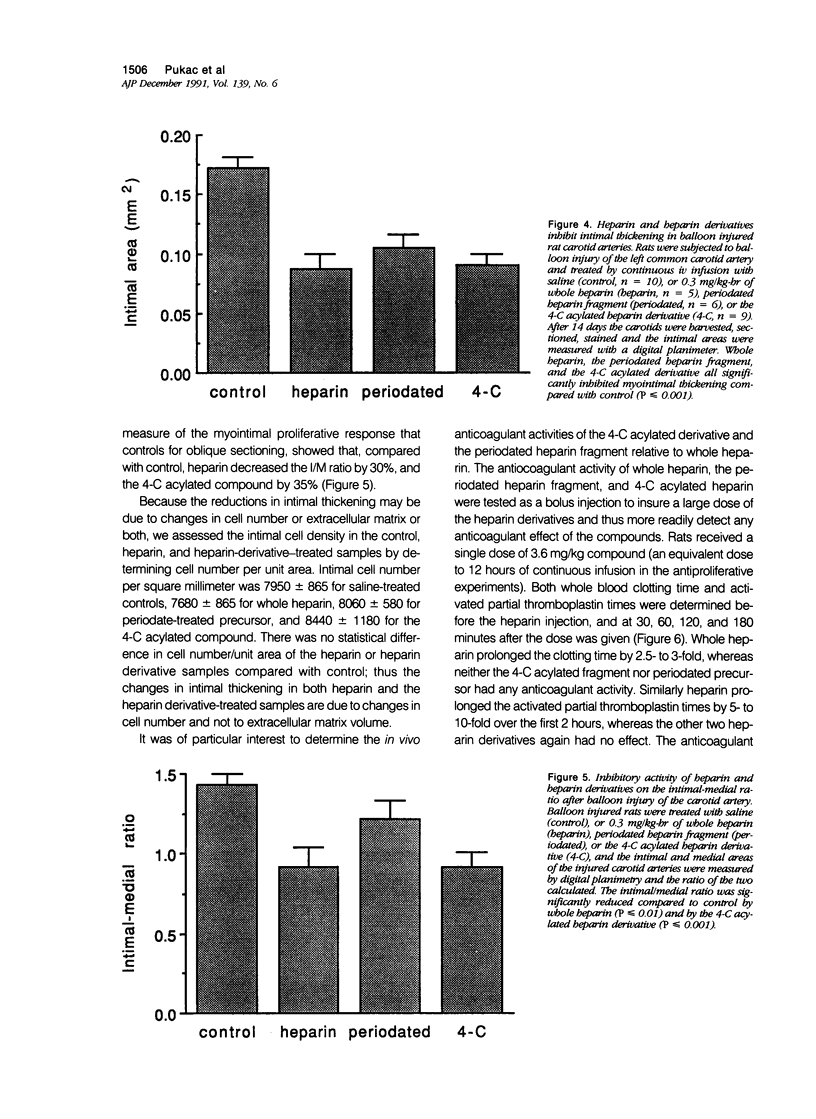
The proliferation of vascular smooth muscle cells (VSMC) is strongly inhibited by whole heparin both in vitro and in vivo. To identify and characterize antiproliferative, but nonanticoagulant heparin derivatives, heparin fragments made by periodate treatment were produced and acylated with 2-, 4-, or 6-carbon chain lengths. In culture, the 4- and 6-carbon acylated compounds were more effective than whole heparin in inhibiting serum stimulated VSMC growth at equal mass or approximately equal mean molar concentrations. Further testing was performed in the rat carotid balloon injury model. Myointimal VSMC proliferation produced by balloon catheterization of rat carotid arteries was inhibited by the 4-carbon acylated compound as effectively as heparin at the same mass dose. Importantly, unlike heparin, the 4-carbon acylated compound had no anticoagulant effect in vivo. These experiments suggest nonanticoagulant, acylated heparin derivatives may have a pharmacologic role in preventing myointimal proliferative lesions that are responsible for failures of vascular surgeries and angioplasties.
Full text
PDF


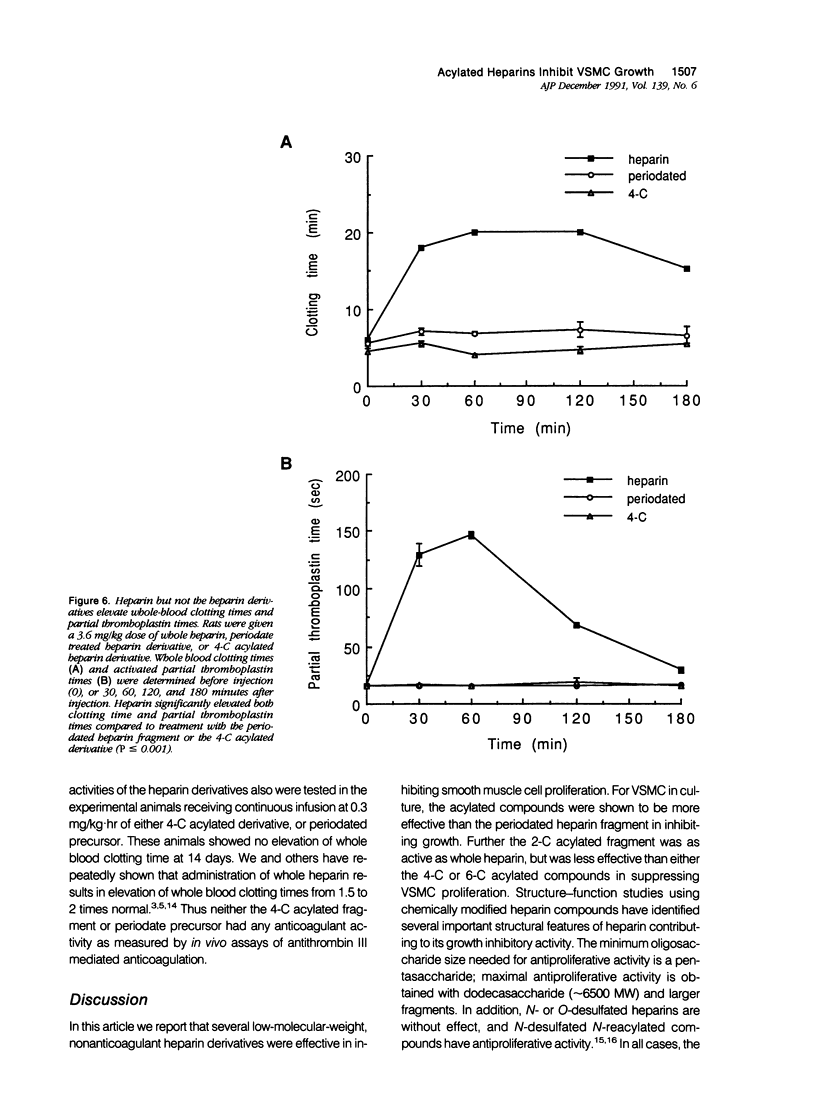


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson J. B., Forman M. B., Vaughn W. K., Robinowitz M., McAllister H. A., Virmani R. Morphologic changes in long-term saphenous vein bypass grafts. Chest. 1985 Sep;88(3):341–348. doi: 10.1378/chest.88.3.341. [DOI] [PubMed] [Google Scholar]
- Beatt K. J., Serruys P. W., Hugenholtz P. G. Restenosis after coronary angioplasty: new standards for clinical studies. J Am Coll Cardiol. 1990 Feb;15(2):491–498. doi: 10.1016/s0735-1097(10)80081-6. [DOI] [PubMed] [Google Scholar]
- Castellot J. J., Jr, Addonizio M. L., Rosenberg R., Karnovsky M. J. Cultured endothelial cells produce a heparinlike inhibitor of smooth muscle cell growth. J Cell Biol. 1981 Aug;90(2):372–379. doi: 10.1083/jcb.90.2.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellot J. J., Jr, Choay J., Lormeau J. C., Petitou M., Sache E., Karnovsky M. J. Structural determinants of the capacity of heparin to inhibit the proliferation of vascular smooth muscle cells. II. Evidence for a pentasaccharide sequence that contains a 3-O-sulfate group. J Cell Biol. 1986 May;102(5):1979–1984. doi: 10.1083/jcb.102.5.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellot J. J., Jr, Hoover R. L., Harper P. A., Karnovsky M. J. Heparin and glomerular epithelial cell-secreted heparin-like species inhibit mesangial-cell proliferation. Am J Pathol. 1985 Sep;120(3):427–435. [PMC free article] [PubMed] [Google Scholar]
- Casu B. Structure of heparin and heparin fragments. Ann N Y Acad Sci. 1989;556:1–17. doi: 10.1111/j.1749-6632.1989.tb22485.x. [DOI] [PubMed] [Google Scholar]
- Chomette G., Auriol M., Cabrol C. Chronic rejection in human heart transplantation. J Heart Transplant. 1988 Jul-Aug;7(4):292–297. [PubMed] [Google Scholar]
- Clowes A. W., Clowes M. M. Kinetics of cellular proliferation after arterial injury. II. Inhibition of smooth muscle growth by heparin. Lab Invest. 1985 Jun;52(6):611–616. [PubMed] [Google Scholar]
- Clowes A. W., Clowes M. M. Kinetics of cellular proliferation after arterial injury. IV. Heparin inhibits rat smooth muscle mitogenesis and migration. Circ Res. 1986 Jun;58(6):839–845. doi: 10.1161/01.res.58.6.839. [DOI] [PubMed] [Google Scholar]
- Clowes A. W., Karnowsky M. J. Suppression by heparin of smooth muscle cell proliferation in injured arteries. Nature. 1977 Feb 17;265(5595):625–626. doi: 10.1038/265625a0. [DOI] [PubMed] [Google Scholar]
- Diamond J. R., Karnovsky M. J. Nonanticoagulant protective effect of heparin in chronic aminonucleoside nephrosis. Ren Physiol. 1986;9(6):366–374. doi: 10.1159/000173102. [DOI] [PubMed] [Google Scholar]
- Edelman E. R., Adams D. H., Karnovsky M. J. Effect of controlled adventitial heparin delivery on smooth muscle cell proliferation following endothelial injury. Proc Natl Acad Sci U S A. 1990 May;87(10):3773–3777. doi: 10.1073/pnas.87.10.3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales C. A., Kradin R. L., Brandstetter R. D., Zhu Y. J. Impairment of hypoxic pulmonary artery remodeling by heparin in mice. Am Rev Respir Dis. 1983 Oct;128(4):747–751. doi: 10.1164/arrd.1983.128.4.747. [DOI] [PubMed] [Google Scholar]
- Hoover R. L., Rosenberg R., Haering W., Karnovsky M. J. Inhibition of rat arterial smooth muscle cell proliferation by heparin. II. In vitro studies. Circ Res. 1980 Oct;47(4):578–583. doi: 10.1161/01.res.47.4.578. [DOI] [PubMed] [Google Scholar]
- Ip J. H., Fuster V., Badimon L., Badimon J., Taubman M. B., Chesebro J. H. Syndromes of accelerated atherosclerosis: role of vascular injury and smooth muscle cell proliferation. J Am Coll Cardiol. 1990 Jun;15(7):1667–1687. doi: 10.1016/0735-1097(90)92845-s. [DOI] [PubMed] [Google Scholar]
- Lippman M. M., Mathews M. B. Heparins: varying effects on cell proliferation in vitro and lack of correlation with anticoagulant activity. Fed Proc. 1977 Jan;36(1):55–59. [PubMed] [Google Scholar]
- Rosenberg R. D. Role of heparin and heparinlike molecules in thrombosis and atherosclerosis. Fed Proc. 1985 Feb;44(2):404–409. [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Snow A. D., Bolender R. P., Wight T. N., Clowes A. W. Heparin modulates the composition of the extracellular matrix domain surrounding arterial smooth muscle cells. Am J Pathol. 1990 Aug;137(2):313–330. [PMC free article] [PubMed] [Google Scholar]
- Somogyi P., Takagi H. A note on the use of picric acid-paraformaldehyde-glutaraldehyde fixative for correlated light and electron microscopic immunocytochemistry. Neuroscience. 1982 Jul;7(7):1779–1783. doi: 10.1016/0306-4522(82)90035-5. [DOI] [PubMed] [Google Scholar]
- Wright T. C., Jr, Castellot J. J., Jr, Petitou M., Lormeau J. C., Choay J., Karnovsky M. J. Structural determinants of heparin's growth inhibitory activity. Interdependence of oligosaccharide size and charge. J Biol Chem. 1989 Jan 25;264(3):1534–1542. [PubMed] [Google Scholar]