Abstract
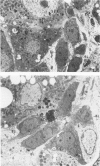
Numerous hepatic cell lineage pathways have been proposed for the development of hepatocarcinogensis induced by chemical carcinogens in rats. The roles of bile ductule cells and hepatocytes in the development of carcinogenesis were investigated using light and electron microscopic procedures to detect differences in morphology and in the phenotypic expression of antigens that are associated with each cell type. In early stages of hepatocarcinogenesis (4-10 weeks after initiation of feeding of a choline-deficient ethionine containing diet), both bile ductulelike (BDL) cells and hepatocytes were seen in mitosis. At the light microscope level, BDL cells showed intense cytoplasmic pyronin (RNA) staining and were positive for the antigens defined by monoclonal antibody 270.38 (bile ductule cells and "oval" cell marker) and glutathione-S-transferase (Yp isoform), whereas hepatocytes were positive for the antigens defined by monoclonal antibodies 270.26 and 258.26 (liver parenchymal cell markers), catalase activity (peroxisome marker) and adenosine triphospatase activity (bile canalicular marker). The authors frequently encountered BDL cells and hepatocytes in close proximity. Ultrastructural examination showed extensive plasma membrane appositions between a subset of BDL cells and hepatocytes. Desmosome structures, tight junctions, microvilli interdigitations and ATPase-positive bile canalicularlike structures were present along the contiguous plasma membrane domains of BDL cells and hepatocytes. Many of the BDL cells attached to hepatocytes were also attached to other BDL cells that had retained a basal lamina. In many cases, BDL cells connected to both hepatocytes and other BDL cells were no longer completely surrounded by basal lamina and had acquired a dual polarity as a consequence of their sharing apical and lateral membrane domains with both BDL cells and hepatocytes. BDL cells showed increased numbers of microperoxisomes (catalase positive organelles) and numerous free ribosomes. Hepatocytes showed a prominent development of the smooth endoplasmic reticulum, a feature prominent in hepatocytes within hyperplastic nodules. Since BDL cells and hepatocytes proliferate and BDL cells and hepatocytes develop intercellular junction sites, the authors propose that both cell types in early stages of carcinogenesis have the capacity to enter the cell lineage pathway leading to the development of hepatocarcinoma. Furthermore, the finding that BDL cells and hepatocytes form multiple attachment sites at the level of the plasma membrane, suggests the possibility that at some stage convergence of separate hepatic cell pathways may occur.
Full text
PDF

















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braun L., Goyette M., Yaswen P., Thompson N. L., Fausto N. Growth in culture and tumorigenicity after transfection with the ras oncogene of liver epithelial cells from carcinogen-treated rats. Cancer Res. 1987 Aug 1;47(15):4116–4124. [PubMed] [Google Scholar]
- Dunsford H. A., Karnasuta C., Hunt J. M., Sell S. Different lineages of chemically induced hepatocellular carcinoma in rats defined by monoclonal antibodies. Cancer Res. 1989 Sep 1;49(17):4894–4900. [PubMed] [Google Scholar]
- Eriksson L. C., Sharma R. N., Roomi M. W., Ho R. K., Farber E., Murray R. K. A characteristic electrophoretic pattern of cytosolic polypeptides from hepatocyte nodules generated during liver carcinogenesis in several models. Biochem Biophys Res Commun. 1983 Dec 28;117(3):740–745. doi: 10.1016/0006-291x(83)91659-5. [DOI] [PubMed] [Google Scholar]
- Evarts R. P., Nagy P., Marsden E., Thorgeirsson S. S. A precursor-product relationship exists between oval cells and hepatocytes in rat liver. Carcinogenesis. 1987 Nov;8(11):1737–1740. doi: 10.1093/carcin/8.11.1737. [DOI] [PubMed] [Google Scholar]
- Evarts R. P., Nagy P., Marsden E., Thorgeirsson S. S. In situ hybridization studies on expression of albumin and alpha-fetoprotein during the early stage of neoplastic transformation in rat liver. Cancer Res. 1987 Oct 15;47(20):5469–5475. [PubMed] [Google Scholar]
- Evarts R. P., Nagy P., Nakatsukasa H., Marsden E., Thorgeirsson S. S. In vivo differentiation of rat liver oval cells into hepatocytes. Cancer Res. 1989 Mar 15;49(6):1541–1547. [PubMed] [Google Scholar]
- Evarts R. P., Nakatsukasa H., Marsden E. R., Hsia C. C., Dunsford H. A., Thorgeirsson S. S. Cellular and molecular changes in the early stages of chemical hepatocarcinogenesis in the rat. Cancer Res. 1990 Jun 1;50(11):3439–3444. [PubMed] [Google Scholar]
- Farber E. The multistep nature of cancer development. Cancer Res. 1984 Oct;44(10):4217–4223. [PubMed] [Google Scholar]
- Faris R. A., Monfils B. A., Dunsford H. A., Hixson D. C. Antigenic relationship between oval cells and a subpopulation of hepatic foci, nodules, and carcinomas induced by the "resistant hepatocyte" model system. Cancer Res. 1991 Feb 15;51(4):1308–1317. [PubMed] [Google Scholar]
- GRISHAM J. W., PORTA E. A. ORIGIN AND FATE OF PROLIFERATED HEPATIC DUCTAL CELLS IN THE RAT: ELECTRON MICROSCOPIC AND AUTORADIOGRAPHIC STUDIES. Exp Mol Pathol. 1964 Jun;86:242–261. doi: 10.1016/0014-4800(64)90057-7. [DOI] [PubMed] [Google Scholar]
- Germain L., Noël M., Gourdeau H., Marceau N. Promotion of growth and differentiation of rat ductular oval cells in primary culture. Cancer Res. 1988 Jan 15;48(2):368–378. [PubMed] [Google Scholar]
- Goyette M., Faris R., Braun L., Hixson D., Fausto N. Expression of hepatocyte and oval cell antigens in hepatocellular carcinomas produced by oncogene-transfected liver epithelial cells. Cancer Res. 1990 Aug 1;50(15):4809–4817. [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Hayner N. T., Braun L., Yaswen P., Brooks M., Fausto N. Isozyme profiles of oval cells, parenchymal cells, and biliary cells isolated by centrifugal elutriation from normal and preneoplastic livers. Cancer Res. 1984 Jan;44(1):332–338. [PubMed] [Google Scholar]
- Hixson D. C., Allison J. P. Monoclonal antibodies recognizing oval cells induced in the liver of rats by N-2-fluorenylacetamide or ethionine in a choline-deficient diet. Cancer Res. 1985 Aug;45(8):3750–3760. [PubMed] [Google Scholar]
- Hixson D. C., Faris R. A., Thompson N. L. An antigenic portrait of the liver during carcinogenesis. Pathobiology. 1990;58(2):65–77. doi: 10.1159/000163565. [DOI] [PubMed] [Google Scholar]
- Lazarow P. B. Rat liver peroxisomes catalyze the beta oxidation of fatty acids. J Biol Chem. 1978 Mar 10;253(5):1522–1528. [PubMed] [Google Scholar]
- Lombardi B. Effects of choline deficiency on rat hepatocytes. Fed Proc. 1971 Jan-Feb;30(1):139–142. [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Novikoff A. B., Novikoff P. M., Davis C., Quintana N. Studies on microperoxisomes. II. A cytochemical method for light and electron microscopy. J Histochem Cytochem. 1972 Dec;20(12):1006–1023. doi: 10.1177/20.12.1006. [DOI] [PubMed] [Google Scholar]
- Novikoff A. B., Novikoff P. M., Davis C., Quintana N. Studies on microperoxisomes. V. Are microperoxisomes ubiquitous in mammalian cells? J Histochem Cytochem. 1973 Aug;21(8):737–755. doi: 10.1177/21.8.737. [DOI] [PubMed] [Google Scholar]
- Novikoff A. B., Novikoff P. M., Stockert R. J., Becker F. F., Yam A., Poruchynsky M. S., Levin W., Thomas P. E. Immunocytochemical localization of epoxide hydrase in hyperplastic nodules induced in rat liver by 2-acetylaminofluorene. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5207–5211. doi: 10.1073/pnas.76.10.5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novikoff P. M., La Russo N. F., Novikoff A. B., Stockert R. J., Yam A., Le Sage G. D. Immunocytochemical localization of lysosomal beta-galactosidase in rat liver. J Cell Biol. 1983 Nov;97(5 Pt 1):1559–1565. doi: 10.1083/jcb.97.5.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novikoff P. M., Novikoff A. B. Peroxisomes in absorptive cells of mammalian small intestine. J Cell Biol. 1972 May;53(2):532–560. doi: 10.1083/jcb.53.2.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novikoff P. M., Novikoff A. B., Quintana N., Davis C. Studies on microperoxisomes. 3. Observations on human and rat hepatocytes. J Histochem Cytochem. 1973 Jun;21(6):540–558. doi: 10.1177/21.6.540. [DOI] [PubMed] [Google Scholar]
- Rushmore T. H., Harris L., Nagai M., Sharma R. N., Hayes M. A., Cameron R. G., Murray R. K., Farber E. Purification and characterization of P-52 (glutathione S-transferase-P or 7-7) from normal liver and putative preneoplastic liver nodules. Cancer Res. 1988 May 15;48(10):2805–2812. [PubMed] [Google Scholar]
- Sato K. Glutathione S-transferases and hepatocarcinogenesis. Jpn J Cancer Res. 1988 May;79(5):556–572. doi: 10.1111/j.1349-7006.1988.tb00022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell S., Dunsford H. A. Evidence for the stem cell origin of hepatocellular carcinoma and cholangiocarcinoma. Am J Pathol. 1989 Jun;134(6):1347–1363. [PMC free article] [PubMed] [Google Scholar]
- Shinozuka H., Lombardi B., Sell S., Iammarino R. M. Early histological and functional alterations of ethionine liver carcinogenesis in rats fed a choline-deficient diet. Cancer Res. 1978 Apr;38(4):1092–1098. [PubMed] [Google Scholar]
- Sirica A. E., Mathis G. A., Sano N., Elmore L. W. Isolation, culture, and transplantation of intrahepatic biliary epithelial cells and oval cells. Pathobiology. 1990;58(1):44–64. doi: 10.1159/000163564. [DOI] [PubMed] [Google Scholar]
- Spelman L. H., Thompson N. L., Fausto N., Miller K. R. A structural analysis of gap and tight junctions in the rat liver during a dietary treatment that induces oval cell proliferation. Am J Pathol. 1986 Nov;125(2):379–392. [PMC free article] [PubMed] [Google Scholar]
- Teebor G. W., Becker F. F. Regression and persistence of hyperplastic hepatic nodules induced by N-2-Fluorenylacetamide and their relationship to hepatocarcinogenesis. Cancer Res. 1971 Jan;31(1):1–3. [PubMed] [Google Scholar]
- Tsao M. S., Smith J. D., Nelson K. G., Grisham J. W. A diploid epithelial cell line from normal adult rat liver with phenotypic properties of 'oval' cells. Exp Cell Res. 1984 Sep;154(1):38–52. doi: 10.1016/0014-4827(84)90666-9. [DOI] [PubMed] [Google Scholar]
- WACHSTEIN M., MEISEL E. Histochemistry of hepatic phosphatases of a physiologic pH; with special reference to the demonstration of bile canaliculi. Am J Clin Pathol. 1957 Jan;27(1):13–23. doi: 10.1093/ajcp/27.1.13. [DOI] [PubMed] [Google Scholar]
- Yaswen P., Hayner N. T., Fausto N. Isolation of oval cells by centrifugal elutriation and comparison with other cell types purified from normal and preneoplastic livers. Cancer Res. 1984 Jan;44(1):324–331. [PubMed] [Google Scholar]