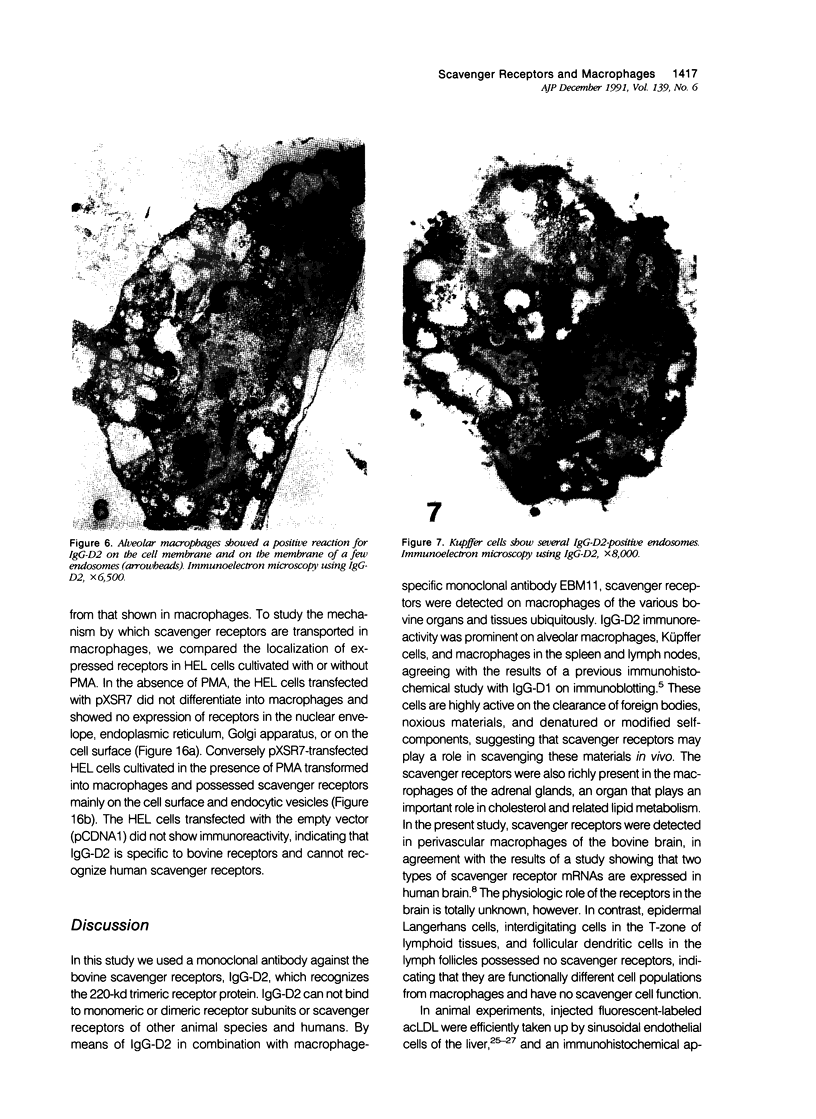
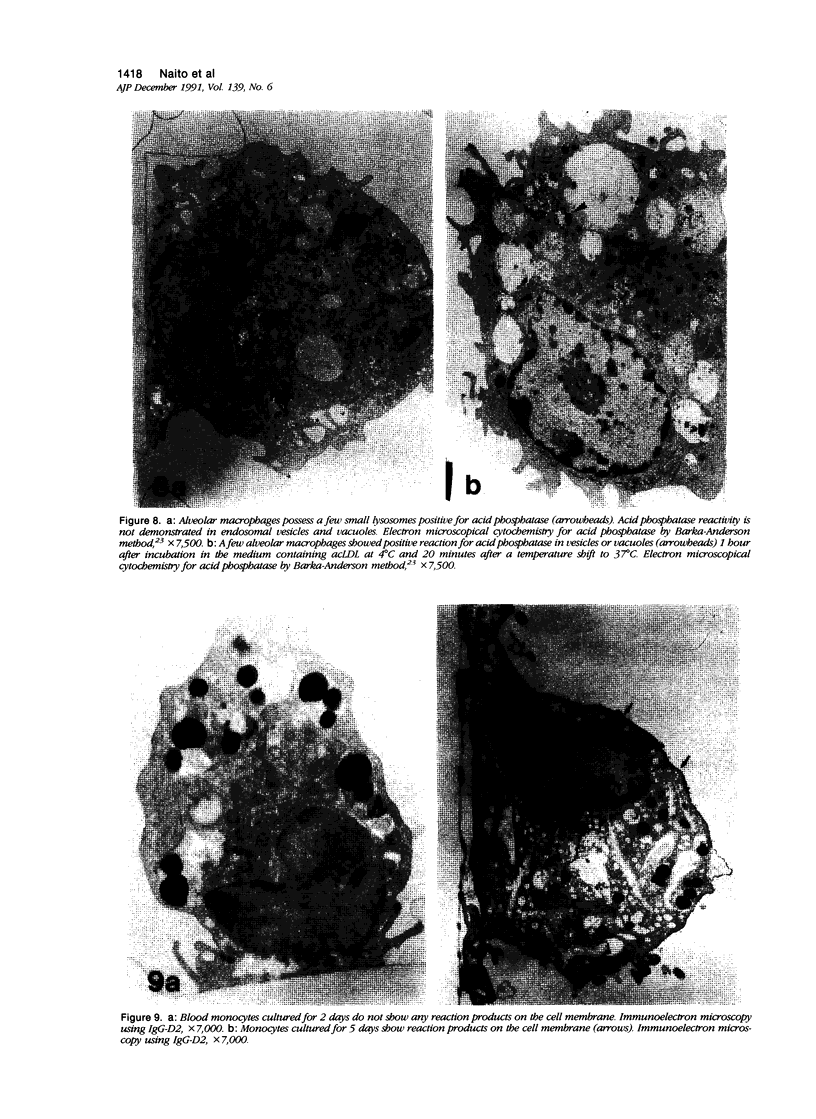
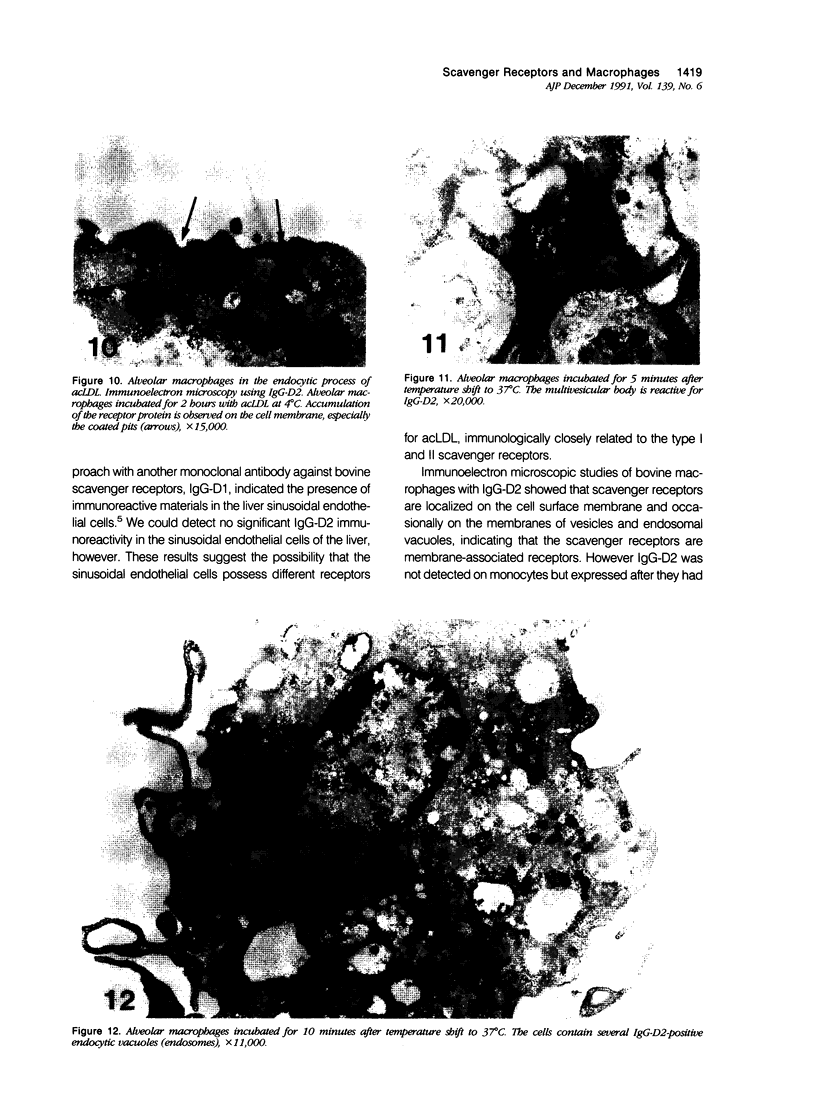
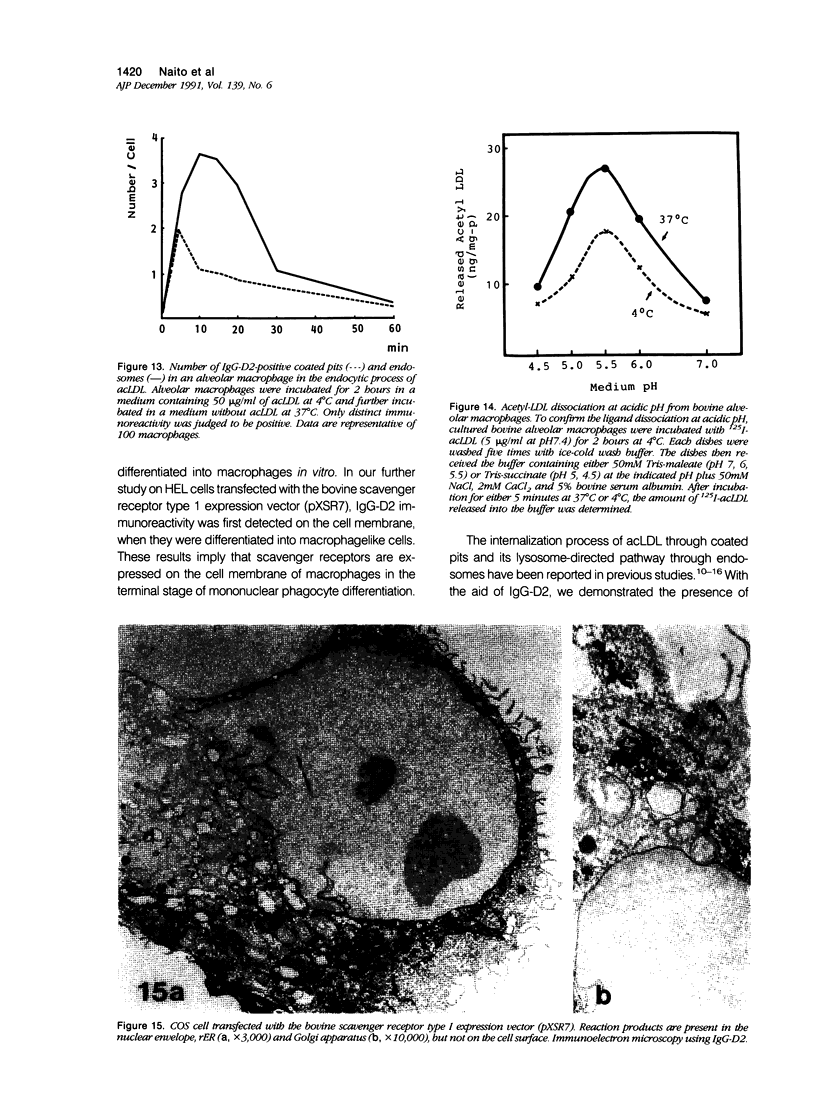
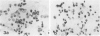Abstract
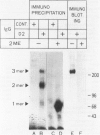
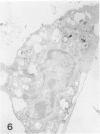
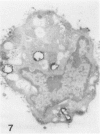
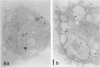
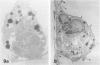
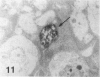
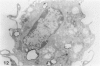
Macrophage scavenger receptors are trimeric membrane proteins implicated in the pathologic deposition of cholesterol in atherogenesis. The authors have studied the tissue distribution and intracellular localization of bovine scavenger receptors using monoclonal antibovine receptor antibody IgG-D2. The receptor proteins were detectable in macrophages of various organs and tissues, particularly Kupffer cells, alveolar macrophages, and macrophages in the spleen and lymph nodes. In the brain, perivascular macrophages were immunoreactive with IgG-D2. Fibroblasts, endothelial cells, smooth muscle cells, and dendritic cells such as epidermal Langerhans cells, interdigitating cells, or follicular dendritic cells, however, showed no immunoreactivity to IgG-D2. Immunoelectron microscopy showed localization of reaction products for these receptors on the cell surface, vesicles, and endosomes of macrophages. Transient expression of bovine scavenger receptors on cultured cells shows that scavenger receptors are mainly expressed in the endoplasmic reticulum, nuclear envelope, and Golgi apparatus of nonmacrophage cells and moved to the cell surface and endosomes of macrophagelike cells. These results indicate that efficient intracellular transport of scavenger receptors in macrophages is mediated by a macrophage-specific transport system.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahamson D. R., Fearon D. T. Endocytosis of the C3b receptor of complement within coated pits in human polymorphonuclear leukocytes and monocytes. Lab Invest. 1983 Feb;48(2):162–168. [PubMed] [Google Scholar]
- Bielefeldt-Ohmann H., Sabara M., Lawman M. J., Griebel P., Babiuk L. A. A monoclonal antibody detects macrophage maturation antigen which appears independently of class II antigen expression. Reactivity of monoclonal EBM11 with bovine macrophages. J Immunol. 1988 Apr 1;140(7):2201–2209. [PubMed] [Google Scholar]
- Blomhoff R., Drevon C. A., Eskild W., Helgerud P., Norum K. R., Berg T. Clearance of acetyl low density lipoprotein by rat liver endothelial cells. Implications for hepatic cholesterol metabolism. J Biol Chem. 1984 Jul 25;259(14):8898–8903. [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Atherosclerosis. Scavenging for receptors. Nature. 1990 Feb 8;343(6258):508–509. doi: 10.1038/343508a0. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Lipoprotein metabolism in the macrophage: implications for cholesterol deposition in atherosclerosis. Annu Rev Biochem. 1983;52:223–261. doi: 10.1146/annurev.bi.52.070183.001255. [DOI] [PubMed] [Google Scholar]
- Davis C. G., Goldstein J. L., Südhof T. C., Anderson R. G., Russell D. W., Brown M. S. Acid-dependent ligand dissociation and recycling of LDL receptor mediated by growth factor homology region. Nature. 1987 Apr 23;326(6115):760–765. doi: 10.1038/326760a0. [DOI] [PubMed] [Google Scholar]
- Dickson R. B., Nicolas J. C., Willingham M. C., Pastan I. Internalization of alpha 2 macroglobulin in receptosomes. Studies with monovalent electron microscopic markers. Exp Cell Res. 1981 Apr;132(2):488–493. doi: 10.1016/0014-4827(81)90127-0. [DOI] [PubMed] [Google Scholar]
- Freeman M., Ashkenas J., Rees D. J., Kingsley D. M., Copeland N. G., Jenkins N. A., Krieger M. An ancient, highly conserved family of cysteine-rich protein domains revealed by cloning type I and type II murine macrophage scavenger receptors. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8810–8814. doi: 10.1073/pnas.87.22.8810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda S., Horiuchi S., Tomita K., Murakami M., Morino Y., Takahashi K. Acetylated low-density lipoprotein is endocytosed through coated pits by rat peritoneal macrophages. Virchows Arch B Cell Pathol Incl Mol Pathol. 1986;52(1):1–13. doi: 10.1007/BF02889945. [DOI] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Henson D. A., St Clair R. W., Lewis J. C. Morphological characterization of beta-VLDL and acetylated-LDL binding and internalization by cultured pigeon monocytes. Exp Mol Pathol. 1989 Dec;51(3):243–263. doi: 10.1016/0014-4800(89)90023-3. [DOI] [PubMed] [Google Scholar]
- Kiss A. L., Röhlich P. Receptor-mediated pinocytosis of IgG and immune complex in rat peritoneal macrophages: an electron microscopic study. Eur J Cell Biol. 1984 May;34(1):88–95. [PubMed] [Google Scholar]
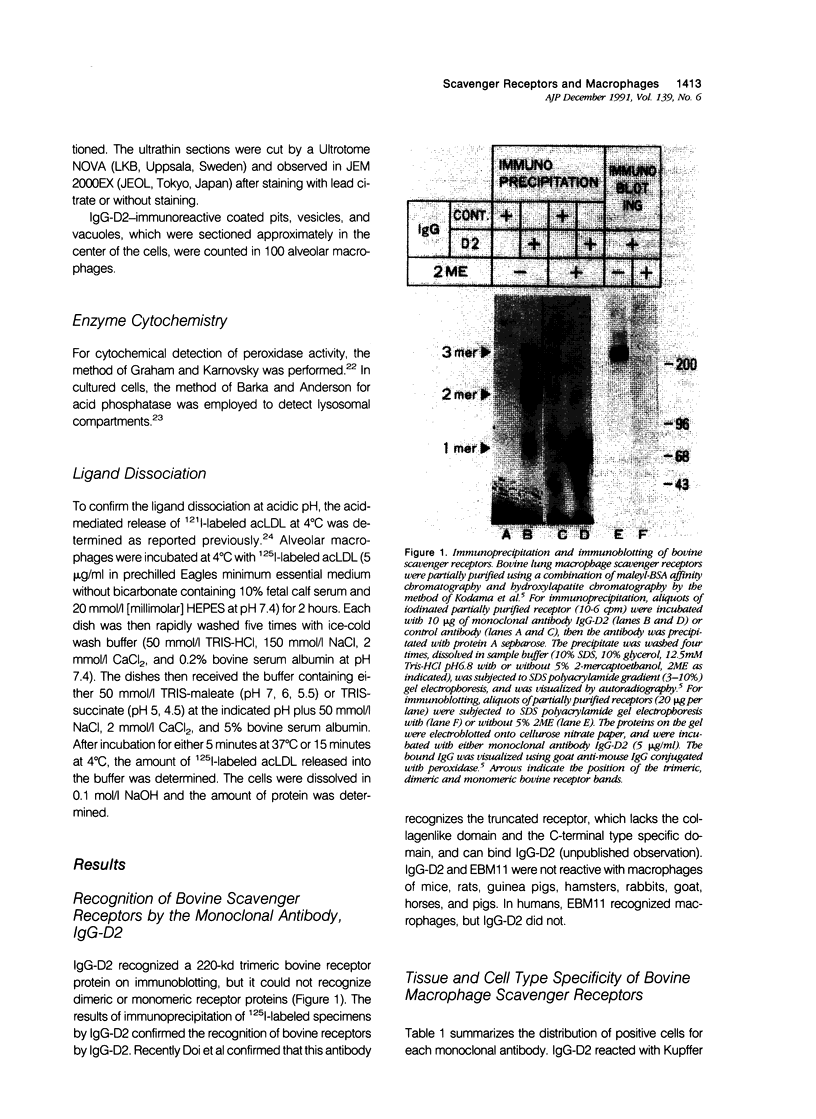
- Kodama T., Freeman M., Rohrer L., Zabrecky J., Matsudaira P., Krieger M. Type I macrophage scavenger receptor contains alpha-helical and collagen-like coiled coils. Nature. 1990 Feb 8;343(6258):531–535. doi: 10.1038/343531a0. [DOI] [PubMed] [Google Scholar]
- Kodama T., Reddy P., Kishimoto C., Krieger M. Purification and characterization of a bovine acetyl low density lipoprotein receptor. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9238–9242. doi: 10.1073/pnas.85.23.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto A., Naito M., Itakura H., Ikemoto S., Asaoka H., Hayakawa I., Kanamori H., Aburatani H., Takaku F., Suzuki H. Human macrophage scavenger receptors: primary structure, expression, and localization in atherosclerotic lesions. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9133–9137. doi: 10.1073/pnas.87.23.9133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Mommaas-Kienhuis A. M., Krijbolder L. H., Van Hinsbergh V. W., Daems W. T., Vermeer B. J. Visualization of binding and receptor-mediated uptake of low density lipoproteins by human endothelial cells. Eur J Cell Biol. 1985 Mar;36(2):201–208. [PubMed] [Google Scholar]
- Mommaas-Kienhuis A. M., van der Schroeff J. G., Wijsman M. C., Daems W. T., Vermeer B. J. Conjugates of colloidal gold with native and acetylated low density lipoproteins for ultrastructural investigations on receptor-mediated endocytosis by cultured human monocyte-derived macrophages. Histochemistry. 1985;83(1):29–35. doi: 10.1007/BF00495296. [DOI] [PubMed] [Google Scholar]
- Nagelkerke J. F., Barto K. P., van Berkel T. J. In vivo and in vitro uptake and degradation of acetylated low density lipoprotein by rat liver endothelial, Kupffer, and parenchymal cells. J Biol Chem. 1983 Oct 25;258(20):12221–12227. [PubMed] [Google Scholar]
- Pitas R. E., Boyles J., Mahley R. W., Bissell D. M. Uptake of chemically modified low density lipoproteins in vivo is mediated by specific endothelial cells. J Cell Biol. 1985 Jan;100(1):103–117. doi: 10.1083/jcb.100.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter H., Weir L., Leder P. Enhancer-dependent expression of human kappa immunoglobulin genes introduced into mouse pre-B lymphocytes by electroporation. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7161–7165. doi: 10.1073/pnas.81.22.7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robenek H., Schmitz G., Assmann G. Topography and dynamics of receptors for acetylated and malondialdehyde-modified low-density lipoprotein in the plasma membrane of mouse peritoneal macrophages as visualized by colloidal gold in conjunction with surface replicas. J Histochem Cytochem. 1984 Oct;32(10):1017–1027. doi: 10.1177/32.10.6481148. [DOI] [PubMed] [Google Scholar]
- Rohrer L., Freeman M., Kodama T., Penman M., Krieger M. Coiled-coil fibrous domains mediate ligand binding by macrophage scavenger receptor type II. Nature. 1990 Feb 8;343(6258):570–572. doi: 10.1038/343570a0. [DOI] [PubMed] [Google Scholar]
- Steinberg D., Parthasarathy S., Carew T. E., Khoo J. C., Witztum J. L. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989 Apr 6;320(14):915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- Sussman D. J., Milman G. Short-term, high-efficiency expression of transfected DNA. Mol Cell Biol. 1984 Aug;4(8):1641–1643. doi: 10.1128/mcb.4.8.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traber M. G., Defendi V., Kayden H. J. Receptor activities for low-density lipoprotein and acetylated low-density lipoprotein in a mouse macrophage cell line (IC21) and in human monocyte-derived macrophages. J Exp Med. 1981 Dec 1;154(6):1852–1867. doi: 10.1084/jem.154.6.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traber M. G., Kallman B., Kayden H. J. Localization of the binding sites of native and acetylated low-density lipoprotein (LDL) in human monocyte-derived macrophages. Exp Cell Res. 1983 Oct 15;148(2):281–292. doi: 10.1016/0014-4827(83)90152-0. [DOI] [PubMed] [Google Scholar]
- Wall D. A., Wilson G., Hubbard A. L. The galactose-specific recognition system of mammalian liver: the route of ligand internalization in rat hepatocytes. Cell. 1980 Aug;21(1):79–93. doi: 10.1016/0092-8674(80)90116-6. [DOI] [PubMed] [Google Scholar]
- Willingham M. C., Pastan I. H., Sahagian G. G., Jourdian G. W., Neufeld E. F. Morphologic study of the internalization of a lysosomal enzyme by the mannose 6-phosphate receptor in cultured Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6967–6971. doi: 10.1073/pnas.78.11.6967. [DOI] [PMC free article] [PubMed] [Google Scholar]