Abstract
To clarify the relationship between amyloid formation and amyloid precursor protein (APP), the brain sections from eight patients with Alzheimer's disease (AD) and four with Gerstmann-Sträussler Syndrome (GSS) were investigated immunohistochemically by the double-immunostaining method. In AD, most APP-positive senile plaques belong to classical plaques or primitive plaques, whereas in diffuse plaques, APP-positive neuritic components are rarely observed. The authors documented that anti-APP-labeled degenerative neurites surrounding kuru plaques in all four GSS patients. These kuru plaques were verified by double immunostaining using anti-prion protein and anti-APP. The APP-positive structures in kuru plaques were almost identical with those seen in the classical plaques in AD. The authors concluded that APP-positive degenerative neurites are not an early event in the amyloid formation of senile plaques. It is therefore postulated that depositions of beta/A4 and prion proteins are primary events that may involve the surrounding microenvironment and result in the secondary formation of APP-positive degenerative neurites, not specific to AD.
Full text
PDF
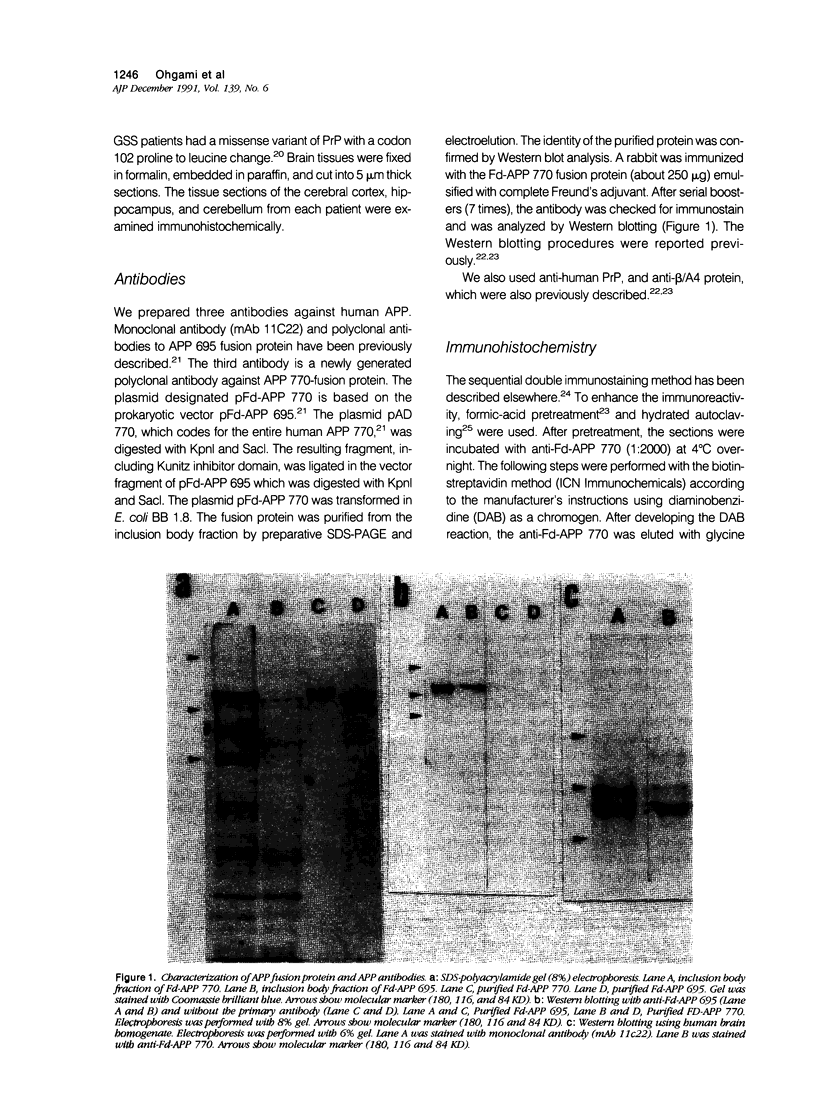

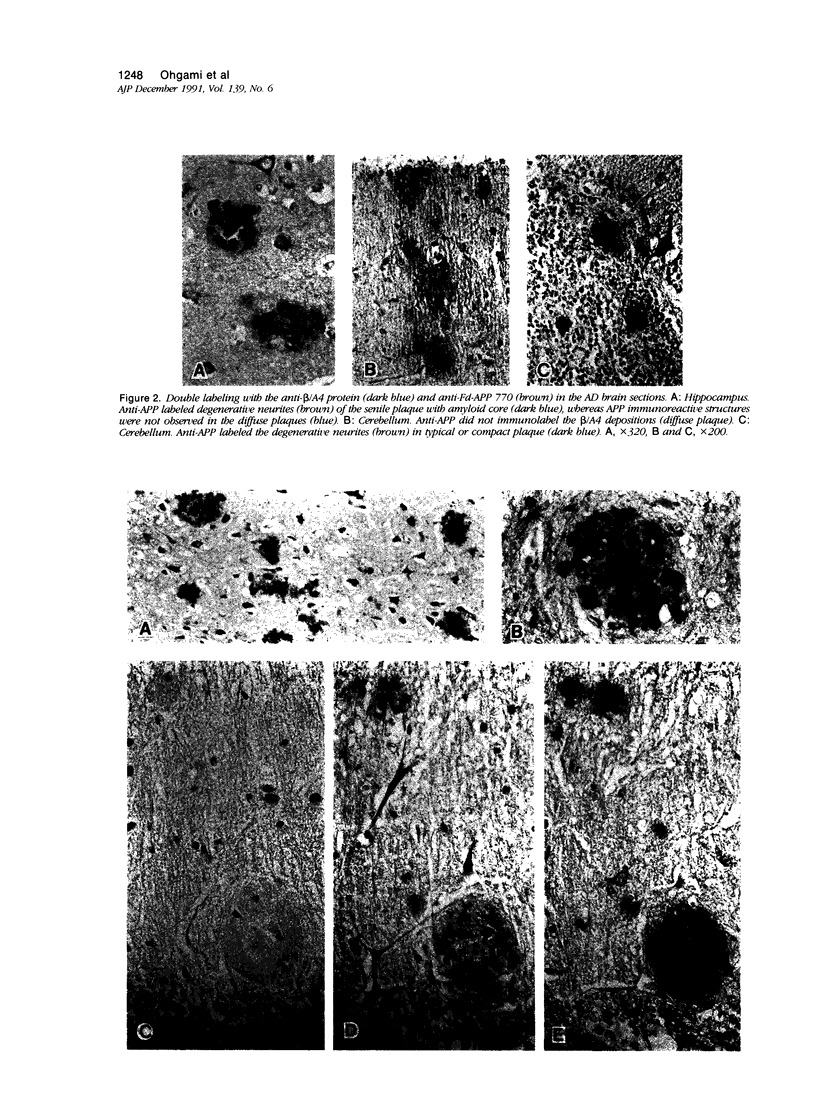


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai H., Lee V. M., Otvos L., Jr, Greenberg B. D., Lowery D. E., Sharma S. K., Schmidt M. L., Trojanowski J. Q. Defined neurofilament, tau, and beta-amyloid precursor protein epitopes distinguish Alzheimer from non-Alzheimer senile plaques. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2249–2253. doi: 10.1073/pnas.87.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brion J. P., Fraser H., Flament-Durand J., Dickinson A. G. Amyloid scrapie plaques in mice, and Alzheimer senile plaques, share common antigens with tau, a microtubule-associated protein. Neurosci Lett. 1987 Jul 9;78(1):113–118. doi: 10.1016/0304-3940(87)90571-4. [DOI] [PubMed] [Google Scholar]
- Brown P. Central nervous system amyloidoses: a comparison of Alzheimer's disease and Creutzfeldt-Jakob disease. Neurology. 1989 Aug;39(8):1103–1105. doi: 10.1212/wnl.39.8.1103. [DOI] [PubMed] [Google Scholar]
- Cole G., Masliah E., Huynh T. V., DeTeresa R., Terry R. D., Okuda C., Saitoh T. An antiserum against amyloid beta-protein precursor detects a unique peptide in Alzheimer brain. Neurosci Lett. 1989 May 22;100(1-3):340–346. doi: 10.1016/0304-3940(89)90710-6. [DOI] [PubMed] [Google Scholar]
- Cras P., Kawai M., Siedlak S., Mulvihill P., Gambetti P., Lowery D., Gonzalez-DeWhitt P., Greenberg B., Perry G. Neuronal and microglial involvement in beta-amyloid protein deposition in Alzheimer's disease. Am J Pathol. 1990 Aug;137(2):241–246. [PMC free article] [PubMed] [Google Scholar]
- Doh-ura K., Tateishi J., Kitamoto T., Sasaki H., Sakaki Y. Creutzfeldt-Jakob disease patients with congophilic kuru plaques have the missense variant prion protein common to Gerstmann-Sträussler syndrome. Ann Neurol. 1990 Feb;27(2):121–126. doi: 10.1002/ana.410270205. [DOI] [PubMed] [Google Scholar]
- Ghiso J., Tagliavini F., Timmers W. F., Frangione B. Alzheimer's disease amyloid precursor protein is present in senile plaques and cerebrospinal fluid: immunohistochemical and biochemical characterization. Biochem Biophys Res Commun. 1989 Aug 30;163(1):430–437. doi: 10.1016/0006-291x(89)92154-2. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Wong C. W. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984 May 16;120(3):885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Soban E. Color modification of diaminobenzidine (DAB) precipitation by metallic ions and its application for double immunohistochemistry. J Histochem Cytochem. 1982 Oct;30(10):1079–1082. doi: 10.1177/30.10.6182185. [DOI] [PubMed] [Google Scholar]
- Ikeda S., Yanagisawa N., Allsop D., Glenner G. G. Evidence of amyloid beta-protein immunoreactive early plaque lesions in Down's syndrome brains. Lab Invest. 1989 Jul;61(1):133–137. [PubMed] [Google Scholar]
- Ishii T., Kametani F., Haga S., Sato M. The immunohistochemical demonstration of subsequences of the precursor of the amyloid A4 protein in senile plaques in Alzheimer's disease. Neuropathol Appl Neurobiol. 1989 Mar-Apr;15(2):135–147. doi: 10.1111/j.1365-2990.1989.tb01216.x. [DOI] [PubMed] [Google Scholar]
- Joachim C. L., Morris J. H., Selkoe D. J. Diffuse senile plaques occur commonly in the cerebellum in Alzheimer's disease. Am J Pathol. 1989 Aug;135(2):309–319. [PMC free article] [PubMed] [Google Scholar]
- Joachim C., Games D., Morris J., Ward P., Frenkel D., Selkoe D. Antibodies to non-beta regions of the beta-amyloid precursor protein detect a subset of senile plaques. Am J Pathol. 1991 Feb;138(2):373–384. [PMC free article] [PubMed] [Google Scholar]
- Kang J., Lemaire H. G., Unterbeck A., Salbaum J. M., Masters C. L., Grzeschik K. H., Multhaup G., Beyreuther K., Müller-Hill B. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987 Feb 19;325(6106):733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Khachaturian Z. S. Diagnosis of Alzheimer's disease. Arch Neurol. 1985 Nov;42(11):1097–1105. doi: 10.1001/archneur.1985.04060100083029. [DOI] [PubMed] [Google Scholar]
- Kitaguchi N., Takahashi Y., Tokushima Y., Shiojiri S., Ito H. Novel precursor of Alzheimer's disease amyloid protein shows protease inhibitory activity. Nature. 1988 Feb 11;331(6156):530–532. doi: 10.1038/331530a0. [DOI] [PubMed] [Google Scholar]
- Kitamoto T., Ogomori K., Tateishi J., Prusiner S. B. Formic acid pretreatment enhances immunostaining of cerebral and systemic amyloids. Lab Invest. 1987 Aug;57(2):230–236. [PubMed] [Google Scholar]
- Kitamoto T., Tateishi J. Immunohistochemical confirmation of Creutzfeldt-Jakob disease with a long clinical course with amyloid plaque core antibodies. Am J Pathol. 1988 Jun;131(3):435–443. [PMC free article] [PubMed] [Google Scholar]
- Kitamoto T., Tateishi J., Sato Y. Immunohistochemical verification of senile and kuru plaques in Creutzfeldt-Jakob disease and the allied disease. Ann Neurol. 1988 Oct;24(4):537–542. doi: 10.1002/ana.410240410. [DOI] [PubMed] [Google Scholar]
- Koo E. H., Sisodia S. S., Archer D. R., Martin L. J., Weidemann A., Beyreuther K., Fischer P., Masters C. L., Price D. L. Precursor of amyloid protein in Alzheimer disease undergoes fast anterograde axonal transport. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1561–1565. doi: 10.1073/pnas.87.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe J., McDermott H., Kenward N., Landon M., Mayer R. J., Bruce M., McBride P., Somerville R. A., Hope J. Ubiquitin conjugate immunoreactivity in the brains of scrapie infected mice. J Pathol. 1990 Sep;162(1):61–66. doi: 10.1002/path.1711620112. [DOI] [PubMed] [Google Scholar]
- Masters C. L., Simms G., Weinman N. A., Multhaup G., McDonald B. L., Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazono M., Iwaki T., Kitamoto T., Kaneko Y., Doh-ura K., Tateishi J. A comparative immunohistochemical study of Kuru and senile plaques with a special reference to glial reactions at various stages of amyloid plaque formation. Am J Pathol. 1991 Sep;139(3):589–598. [PMC free article] [PubMed] [Google Scholar]
- Oesch B., Westaway D., Wälchli M., McKinley M. P., Kent S. B., Aebersold R., Barry R. A., Tempst P., Teplow D. B., Hood L. E. A cellular gene encodes scrapie PrP 27-30 protein. Cell. 1985 Apr;40(4):735–746. doi: 10.1016/0092-8674(85)90333-2. [DOI] [PubMed] [Google Scholar]
- Ogomori K., Kitamoto T., Tateishi J., Sato Y., Suetsugu M., Abe M. Beta-protein amyloid is widely distributed in the central nervous system of patients with Alzheimer's disease. Am J Pathol. 1989 Feb;134(2):243–251. [PMC free article] [PubMed] [Google Scholar]
- Ohgami T., Kitamoto T., Shin R. W., Kaneko Y., Ogomori K., Tateishi J. Increased senile plaques without microglia in Alzheimer's disease. Acta Neuropathol. 1991;81(3):242–247. doi: 10.1007/BF00305864. [DOI] [PubMed] [Google Scholar]
- Perry G., Lipphardt S., Mulvihill P., Kancherla M., Mijares M., Gambetti P., Sharma S., Maggiora L., Cornette J., Lobl T. Amyloid precursor protein in senile plaques of Alzheimer disease. Lancet. 1988 Sep 24;2(8613):746–746. doi: 10.1016/s0140-6736(88)90219-x. [DOI] [PubMed] [Google Scholar]
- Ponte P., Gonzalez-DeWhitt P., Schilling J., Miller J., Hsu D., Greenberg B., Davis K., Wallace W., Lieberburg I., Fuller F. A new A4 amyloid mRNA contains a domain homologous to serine proteinase inhibitors. Nature. 1988 Feb 11;331(6156):525–527. doi: 10.1038/331525a0. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B. Novel proteinaceous infectious particles cause scrapie. Science. 1982 Apr 9;216(4542):136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- Robakis N. K., Ramakrishna N., Wolfe G., Wisniewski H. M. Molecular cloning and characterization of a cDNA encoding the cerebrovascular and the neuritic plaque amyloid peptides. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4190–4194. doi: 10.1073/pnas.84.12.4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin R. W., Iwaki T., Kitamoto T., Tateishi J. Hydrated autoclave pretreatment enhances tau immunoreactivity in formalin-fixed normal and Alzheimer's disease brain tissues. Lab Invest. 1991 May;64(5):693–702. [PubMed] [Google Scholar]
- Shin R. W., Ogomori K., Kitamoto T., Tateishi J. Increased tau accumulation in senile plaques as a hallmark in Alzheimer's disease. Am J Pathol. 1989 Jun;134(6):1365–1371. [PMC free article] [PubMed] [Google Scholar]
- Shoji M., Hirai S., Yamaguchi H., Harigaya Y., Kawarabayashi T. Amyloid beta-protein precursor accumulates in dystrophic neurites of senile plaques in Alzheimer-type dementia. Brain Res. 1990 Mar 26;512(1):164–168. doi: 10.1016/0006-8993(90)91187-l. [DOI] [PubMed] [Google Scholar]
- Tanzi R. E., Gusella J. F., Watkins P. C., Bruns G. A., St George-Hyslop P., Van Keuren M. L., Patterson D., Pagan S., Kurnit D. M., Neve R. L. Amyloid beta protein gene: cDNA, mRNA distribution, and genetic linkage near the Alzheimer locus. Science. 1987 Feb 20;235(4791):880–884. doi: 10.1126/science.2949367. [DOI] [PubMed] [Google Scholar]
- Tanzi R. E., McClatchey A. I., Lamperti E. D., Villa-Komaroff L., Gusella J. F., Neve R. L. Protease inhibitor domain encoded by an amyloid protein precursor mRNA associated with Alzheimer's disease. Nature. 1988 Feb 11;331(6156):528–530. doi: 10.1038/331528a0. [DOI] [PubMed] [Google Scholar]
- Tomlinson B. E., Blessed G., Roth M. Observations on the brains of non-demented old people. J Neurol Sci. 1968 Sep-Oct;7(2):331–356. doi: 10.1016/0022-510x(68)90154-8. [DOI] [PubMed] [Google Scholar]
- Weidemann A., König G., Bunke D., Fischer P., Salbaum J. M., Masters C. L., Beyreuther K. Identification, biogenesis, and localization of precursors of Alzheimer's disease A4 amyloid protein. Cell. 1989 Apr 7;57(1):115–126. doi: 10.1016/0092-8674(89)90177-3. [DOI] [PubMed] [Google Scholar]