Abstract
The nonprotein amino acids γ-aminobutyric acid (GABA) and β-aminobutyric acid (BABA) have known biological effects in animals and plants. Their mode of action has been the object of thorough research in animals but remains unclear in plants. Our objective was to study the mode of action of BABA in the protection of Arabidopis plants against virulent pathogens. BABA protected Arabidopsis against the oomycete pathogen Peronospora parasitica through activation of natural defense mechanisms of the plant such as callose deposition, the hypersensitive response, and the formation of trailing necroses. BABA was still fully protective against P. parasitica in transgenic plants or mutants impaired in the salicylic acid, jasmonic acid, and ethylene signaling pathways. Treatment with BABA did not induce the accumulation of mRNA of the systemic acquired resistance (SAR)-associated PR-1 and the ethylene- and jasmonic acid-dependent PDF1.2 genes. However, BABA potentiated the accumulation of PR-1 mRNA after attack by virulent pathogenic bacteria. As a result, BABA-treated Arabidopsis plants were less diseased compared with the untreated control. In the case of bacteria, BABA protected mutants insensitive to jasmonic acid and ethylene but was not active in plants impaired in the SAR transduction pathway. Thus, BABA protects Arabidopsis against different virulent pathogens by potentiating pathogen-specific plant resistance mechanisms. In addition, we provide evidence that BABA-mediated papilla formation after P. parasitica infection is independent of the SAR signaling pathway.
Plants have evolved numerous, complex defense mechanisms to survive attacks by fungal and microbial pathogens. Plant resistance responses are genetically determined (1), and, in the case of gene-for-gene resistance, they are manifested through the development of a hypersensitive response (HR) (2). The cloning of resistance genes from several plant species has given exciting clues to a better understanding of race-specific resistance (3). In addition to the gene-for-gene resistance, plants have developed inducible defense mechanisms. In this publication, we show that the nonprotein amino acid β-aminobutyric acid (BABA) can induce disease resistance in Arabidopsis independently of known resistance markers. Typically, after attack by a necrotizing pathogen, the plant reacts by developing a long-lasting defense response (SAR) against a broad spectrum of pathogens. SAR is characterized by an early increase in newly synthesized salicylic acid (SA) (4) followed by the activation of genes encoding PR proteins (5). Application of SA and functional analogs of SA, such as 2,6-dichloroisonicotinic acid and benzothiadiazole, correlates with the induction of both PR gene expression and resistance (5, 6). One possible mode of action of SA in pathogen defense is to condition defense reactions leading to a faster response of the plant after pathogen attack (7). Arabidopsis mutants impaired in SAR have helped to understand the signal transduction pathway leading to resistance. Npr1 mutants do not accumulate PR-1 mRNA in response to SA or its functional analogs and are highly susceptible to infection by virulent pathogens (8). Arabidopsis overexpressing a salicylate hydroxylase gene (NahG) have low levels of SA and are unable to undergo SAR (9). Besides SA, the plant hormones jasmonic acid (JA) and ethylene have been shown to be involved in a separate signal transduction pathway providing resistance against distinct pathogens (10–13). The JA- and ethylene-dependent signaling events could be analyzed with mutants such as jar1 and etr1, which exhibit reduced sensitivity to MeJA and altered perception of ethylene, respectively (14, 15), and have been shown to be more susceptible to certain soil-borne pathogens (16, 17).
Nonprotein amino acids such as GABA and BABA have known biological effects in animals and plants. In animals, GABA and glycine are major inhibitory neurotransmitters (18), whereas BABA is a partial agonist of the glycine receptor (19). In plants, GABA is produced as a response to stress (20), and treatments with BABA were shown to provide protection against various pathogens (21–23). However, little is known concerning the mode of action of BABA; some studies report an induction of PR after BABA treatment (24), whereas others state the contrary (25). Thus, the mode of action of BABA remains a matter of controversy. In the present article, we analyze the effect and the mode of action of BABA in Arabidopsis. We show that BABA mediates the conditioning of induced plant defense mechanisms leading to a phenocopy of genetic resistance after infection with a normally virulent pathogen.
Materials and Methods
Biological Material.
The transgenic Arabidopsis thaliana line harboring the NahG gene (9) was obtained from J. Ryals (Novartis, Research Triangle Park, NC). The Columbia (Col-0) ecotype mutants npr1, jar1, and etr1 were provided by X. Dong (Duke University, Durham, NC), P. E. Staswick (University of Nebraska, Lincoln, NE), and the Nottingham Arabidopsis Stock Center, respectively. Arabidopsis accessions Columbia (Col-0) and Wassilewskija (WS) were obtained from Lehle Seeds (Round Rock, TX). Plants were grown in a steam-sterilized soil mix of commercial potting soil/perlite (3:1) at 22°C day, 18°C night temperature with 12 h light per 24 h. Conservation procedures of Peronospora parasitica have been described previously (26). Strain DC 3000 of Pseudomonas syringae pv tomato (Pst DC 3000) and the isogenic strain carrying the avirulence gene avrRpt2 (27) were cultivated at 28°C, 220 rpm in King's medium B (28) containing rifampicin for selection.
Plant Inoculation and Treatment.
P. parasitica was inoculated by spraying until shortly before droplet run-off occurred with a suspension of 105 conidia/ml of water. Plants inoculated with P. parasitica were kept at 20°C in a 12/12-h light/dark cycle. One-hundred percent relative humidity was necessary during the first and last day of the growth cycle to ensure infection and sporulation.
For bacterial inoculation, cells were collected by centrifugation, resuspended in 10 mM MgCl2 at A600 = 0.2, corresponding to a concentration of 108 cfu/ml. Bacteria were then diluted to 105 cfu/ml in 10 mM MgCl2. Titers were determined as follows. Three leaves per plant were infiltrated using a 1-ml syringe without a needle. Each time point represents 24 leaf discs (0.5 cm diameter) from eight different plants. One disk from each plant was pooled, resulting in three groups containing eight leaf discs each. Leaf discs were washed twice with sterile water and homogenized in 10 mM MgCl2. Quantification was done by plating appropriate dilutions on King's B agar containing rifampicin (50 mg/liter). Tissue samples were harvested from inoculated leaves at 0, 1, 2, and 3 days after infiltration.
For P. parasitica infections, pots containing about 30 2- to 3-week-old Arabidopsis plants were soil drenched with indicated chemicals dissolved in water. Five-week-old Arabidopsis plants treated with 16 mg/liter BABA were used for bacterial infiltrations. Treatments were performed 1 day before inoculation with the pathogen, when not otherwise indicated. Only soil-drench treatments were used to avoid formation of necroses observed after spraying because such necroses induce the SAR pathway and mask the primary effect of BABA.
In Vitro Assay.
In vitro assays for antimicrobial BABA activity against pathogenic fungi were evaluated as radial growth of mycelia discs placed onto the middle of agar plates. Growth was determined after several days on potato dextrose agar (PDA; Difco) medium containing BABA at a final concentration of 1000 mg/liter. For bacterial assays, Pst DC 3000 and P. syringae pv maculicola were cultivated in the minimal medium M9 (29). At A600 = 0.4, the culture was divided into two volumes, one containing a final concentration of 1000 mg/liter BABA and the other not. Bacterial growth was determined every hour up to the stationary phase. Each experiment was performed with six replicates.
In Vivo Assay.
To analyze the germination rate of the obligate biotroph P. parasitica, leaves from untreated control and 16 mg/liter BABA soil-drenched Col-0 plants were harvested 60 h after inoculation with P. parasitica isolate NOCO. Plant tissue was destained overnight in ethanol 95% and stained with aniline blue (30). The germination rate was evaluated by determining the number of germinated conidia on 10 leaves per treatment. Experiments were repeated twice with similar results. Observations were performed with a fluorescence microscope with UV filter (BP 340–380 nm, LP 425 nm).
BABA Metabolism Analysis.
Arabidopsis seeds were sterilized and subsequently grown for 6 weeks on half-strength MS (½ MS) medium (31). Plantlets were then transferred to sterile containers with 35 ml of liquid ½ MS medium and 1 μCi of 14C-BABA (1.03 Ci/mol; Novartis, Basel, Switzerland) or 1 μCi 14C-GABA (15.5 Ci/mol, Novartis, Basel, Switzerland), respectively. Plastic support racks were used to avoid direct contact of the leaves with the radioactive solution. After 2 days of incubation, protoplasts were prepared (32) to determine the presence of radioactivity inside the cells. After harvesting, the protoplasts were subjected to a viability test with fluorescein diacetate (33) and counted to make sure that at least 80% of the protoplasts were viable. A further purification step, consisting of centrifuging (30 s, 15,800 × g in a microfuge) the protoplasts through a hydrophobic layer [381 μl of di-butyl phthalate, 119 μl of phthalic acid bis(2-ethyl-hexylester)], was introduced. Protoplasts were then ground directly in the microtubes, the debris spun down, and the supernatant (cell contents) applied to TLC plates. The pellet was washed four times with MCW (MeOH/CHCl3/water, 12:5:3, vol/vol) to yield the membrane fraction. The cell wall fraction was obtained by spinning down the remains after protoplasts had been released and washing them four times in MCW. TLC plates were run in a solution of 1-butanol/acetic acid/water (60:20:20, vol/vol). Amino acids were visualized by spraying with 0.2% ninhydrin in ethanol before heating for 5 min at 140°C. The radioactive amino acids were detected by autoradiography using a Kodak Xomat film.
RNA Gel Blot Analysis.
RNA was extracted from frozen pulverized plant material in a buffer containing 2 M Tris-HCl, pH 8.0/0.5 M EDTA, pH 8.0/20% SDS in a ratio of 1:2:1 (vol/vol) (34) with an equal volume of buffer-saturated phenol/chloroform/isoamyl alcohol (25:24:1, vol/vol). After centrifugation to separate the phases, the RNA from the aqueous phase was precipitated with 1 volume of 6 M LiCl overnight at 4°C. The pellet was then washed with 70% ethanol and resuspended in H2O. Five micrograms of total RNA were separated on a formaldehyde–agarose gel and transferred to a Nylon membrane (Hybond-N, Amersham Pharmacia). The membrane was probed with 32P-labeled cDNA (RadPrime DNA Labeling System, Life Technologies, Paisley, Scotland) encoding pathogenesis-related proteins PR-1 (35) and PDF1.2 (11).
Results
Protection Against P. parasitica.
To test whether the observed protective effect of BABA on crop plants (36) can be extended to Arabidopsis, the plants were treated with BABA or its isomers, α-aminobutyric acid and GABA, 1 day before inoculation with the oomycete P. parasitica, isolate NOCO. This isolate is virulent on A. thaliana accession Col-0 with conidiophores emerging from the leaf 7 days after inoculation (37). Arabidopsis displayed a remarkable selectivity toward aminobutyric acid isomers; only BABA protected against P. parasitica (Table 1). The protection became first apparent at a concentration as low as 8 mg/liter applied in the soil 1 day before inoculation (Table 1). Similar levels of protection were obtained with the virulent isolate EMWA on Arabidopsis accession Wassilewskija (data not shown).
Table 1.
Sporulation of P. parasitica isolate NOCO on Arabidopsis thaliana (Col-0) treated with isomers of aminobutyric acid
| Treatment | Concentration (mg/liter) | Sporulation intensity*
|
||
|---|---|---|---|---|
| 6 dbi‡ | 1 dbi‡ | 6 dpi§ | ||
| BABA | 0 | ++++ | ++++ | ++++ |
| BABA | 8 | + | − | +++ |
| BABA | 16 | − | − | +++ |
| BABA | 32 | ND | − | ND |
| AABA | 32 | ND | ++++ | ND |
| GABA | 32 | ND | +++ | ND |
Sporulation was scored 7 days after inoculation. −, no sporulation; + to ++++, increasing degrees of sporulation. Experiments were repeated four times with similar results. ND, not determined.
Plants were soil-drenched with chemicals before or after inoculation as indicated.
Days before inoculation.
Days postinoculation.
BABA Does Not Act as an Antimicrobial Compound.
The protection attributable to BABA could be the result of a direct antibiotic activity. In vitro tests on various fungi and bacteria showed that this is not the case, even at 1000 mg/liter, a concentration more than 50 times higher than the one used for treating plants (data not shown). The antifungal activity of BABA against the obligate biotroph P. parasitica was also analyzed in an in vivo assay by determining the germination rate of conidia on leaf surfaces of treated Arabidopsis plants. No difference was observed on BABA-treated plants compared with controls (data not shown). In addition, P. parasitica spores were directly incubated in 12 mg/liter BABA and sprayed in this solution on susceptible Arabidopsis plants. Seven days later, sporulation on these plants was similar to control plants sprayed with spores suspended in water only (data not shown). In addition, BABA protected Arabidopsis only when applied before inoculation (Table 1), demonstrating that this chemical has no curative effect once P. parasitica is established in the leaf. Moreover, a study of the metabolism of BABA with labeled molecules showed that BABA is not metabolized, whereas GABA rapidly breaks down. Most of the BABA label was found in the soluble fraction (cell content), and only very little was detected in the cell wall fraction on autoradiograms (data not shown). Therefore, it is very unlikely that protection attributable to BABA is based on a direct antibiotic activity of BABA or its metabolites. Such protection results very likely from an activation of disease resistance mechanisms in the host plant.
Host Defense Reactions After Infection of BABA-Treated Arabidopsis.
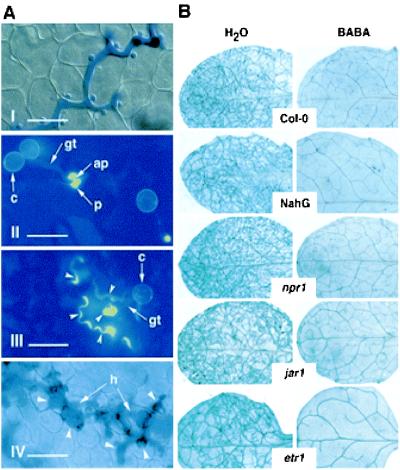
To understand the nature of resistance induced by BABA, cytological observations were performed at infection sites of a virulent P. parasitica isolate in Arabidopsis leaves (Fig. 1A, I). At concentrations of 12 mg/liter or higher, hyphal penetration in the host was completely suppressed. Callose deposits, termed papillae (38), were observed at the site of attempted penetration (Fig. 1A, II). At lower concentrations, cells underwent a phenocopy HR at the site of attack (Fig. 1A, III), and, in some cases, hyphae were able to grow between cells into the leaf tissue. In this situation, the plant often reacted by developing trailing necroses along the growing hyphae (Fig. 1A, IV). These observations all indicate that BABA stimulates the natural defense of the plant by converting phenotypically a compatible into an incompatible host–pathogen interaction.
Figure 1.
BABA-induced resistance in Arabidopsis against P. parasitica. (A) Microscopic aspects of the protective effect of BABA. (I) Growing hyphae in untreated control plant. (Bar = 50 μm.) (II) Callose (yellow) deposition (p, papilla) below the appressoria (ap) at the end of the germ tube (gt) on leaf treated with 12 mg/liter BABA. c, conidium. (Bar = 20 μm.) (III) Phenocopy–HR reaction in plants treated with 8 mg/liter BABA. Callose deposition (arrowheads) around the appressoria and cells undergoing necrosis appear in yellow. (Bar = 20 μm.) (IV) Trailing necrosis (arrowheads) along a growing hypha (h) in a plant treated with 4 mg/liter BABA. (Bar = 50 μm.) Plants were treated with BABA 1 day before inoculation and stained 3 days later with aniline blue for callose observation (Wasserblau Standard Fluka) (30) and Calcofluor White M2 R. S. New (Cyanamid) (44) (II and III) or with lactophenol-trypan blue (I and IV) (45) for fungal structure coloration. Picture IV was taken 6 days after inoculation. (B) Effect of BABA in Arabidopsis lines altered in their response to P. parasitica. Wild-type (Col-0) control, NahG, npr1, jar1, and etr1 plants were treated with water or 12 mg/liter BABA and inoculated with the virulent P. parasitica isolate NOCO. Pictures show leaves stained with lactophenol-trypan blue (45) 7 days after inoculation. Fungal structures and damaged cells are stained in blue. Genotypes and treatments are indicated in the middle and top of the figure, respectively. A representative example for each genotype is shown.
Analysis of the Mode of Action of BABA.
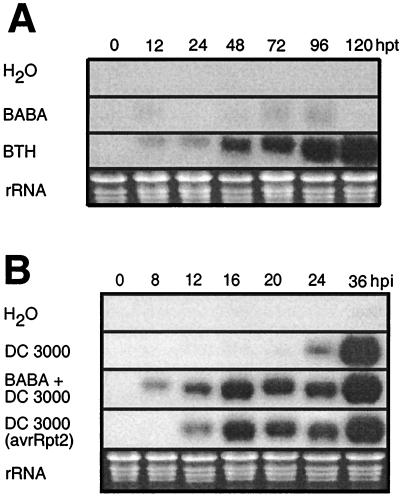
The mode of action of BABA against P. parasitica on Arabidopsis was investigated using transgenic plants or mutants impaired in the signal transduction pathways activated by pathogen infection. We first analyzed whether BABA acts through the SAR transduction pathway. NahG-expressing Arabidopsis plants and npr1 mutants were treated with 12 mg/liter BABA 1 day before inoculation with a virulent isolate of P. parasitica. These plants, like wild-type Col-0, were fully resistant, showing no fungal colonization in the leaf up to 7 days after inoculation. By comparison, the water controls showed an extensive ramification of hyphae in the leaf (Fig. 1B), as well as development of conidiophores and oospores (data not shown). The contribution of JA and ethylene to BABA-induced resistance was analyzed with jar1 and etr1 mutants, respectively. Like the lines deficient in the SAR signaling pathway, these two mutants were completely resistant to P. parasitica infection (Fig. 1B). Thus, despite phenotypical similarities with SAR, BABA-induced resistance against P. parasitica in Arabidopsis is neither dependent on SA accumulation nor on accumulation of PR genes; in addition, it is independent of JA and ethylene perception. Consistent with these observations and in contrast to treatments with the SAR activator benzothiadiazole (39), no PR-1 transcript accumulation after BABA treatment was observed (Fig. 2A). Furthermore, mRNAs for the plant antifungal proteins defensin and thionin, respectively responsive to both JA and ethylene or JA alone (11, 40), were not induced after BABA treatment (data not shown).
Figure 2.
Time course of the expression of PR-1 mRNA in Arabidopsis. (A) Effect of chemical treatments. Total RNA was extracted at various times after being soil drenched with water, 16 mg/liter BABA, or 300 μM benzothiadiazole. hpt, hours posttreatment. (B) Conditioning effect of BABA. Plants were soil drenched with water or 16 mg/liter BABA 1 day before infiltration with bacteria (time 0). Each time point represents nine infected leaves harvested from three different plants. Total RNA was prepared and analyzed by RNA gel blot analysis. Ethidium bromide staining of the RNA gel (rRNA) was used to show equal loading. hpi, hours postinoculation.
Conditioning Effect.
The BABA-induced conversion of a compatible to a phenocopy of an incompatible interaction observed after P. parasitica infection was further explored by analyzing the expression of PR genes after pathogen infection. To induce a strong localized reaction of the plant tissue, we used the virulent bacterium Pst DC 3000 to inoculate plants, and the time-course of the expression of PR-1 mRNA was monitored. BABA treatment conditioned the plant to produce PR-1 mRNA more rapidly. Typically, PR-1 mRNA expression in plants inoculated with Pst DC 3000 was induced 12 h earlier in BABA-treated plants compared with the untreated control. Indeed, the plant reacted as fast as after an infection with avirulent bacteria (Pst DC 3000 avrRpt2) (Fig. 2B). Thus, as observed in the interaction with P. parasitica, BABA treatment mimicked some aspects of genetic resistance through conditioning of plant defense responses.
Protection Against Bacteria.
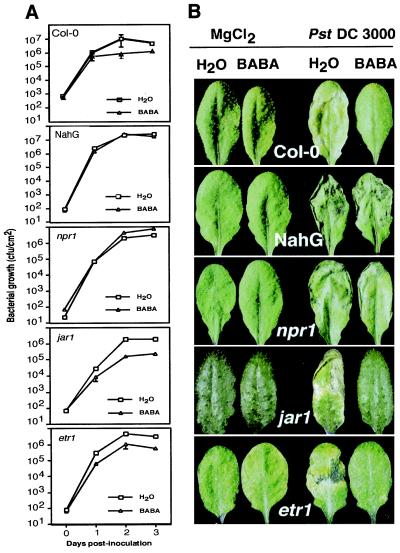
To verify whether this conditioning is effective to protect Arabidopsis against pathogenic bacteria, we infected Col-0 plants with the virulent bacterium Pst DC 3000. As shown in Fig. 3A, BABA treatment resulted in a 10-fold reduction of the bacterial titer and strongly decreased symptoms. Arabidopsis plants pretreated with BABA did not exhibit the typical chlorotic leaf spotting associated with Pst DC 3000 infection (Fig. 3B). Thus, the conditioning effect observed after BABA treatment could be linked to resistance. To further analyze the mode of action of BABA on Arabidopsis against Pst DC 3000, NahG-expressing Arabidopsis plants and npr1 mutants were treated with 16 mg/liter BABA 1 day before inoculation with Pst DC 3000. These plants were not protected, but mutants deficient in the JA or ethylene signaling pathways were protected at a similar level as the wild-type Col-0 (Fig. 3). Consequently, BABA protects Arabidopsis not only against an oomycete but also against a pathogenic bacterium. Moreover, in the case of bacterial infection, the plant protection required PR gene activation or a functional SA signal transduction pathway.
Figure 3.
Protection effect of BABA in Arabidopsis infected with Pst DC 3000. (A) Bacterial growth. Wild-type Col-0, NahG, npr1, jar1, and etr1 leaves were analyzed for bacterial density at different time points after infiltration. Data represent the mean ± SE of three pools stemming from eight replicate samples. Experiments were repeated four times with similar results. (B) Symptoms. Pictures show disease symptoms 3 days after infiltration in wild-type Col-0, NahG, npr1, jar1, and etr1 plants. Treatments are indicated at the top of the figure. Representative examples are shown.
Discussion
Nonprotein amino acids are secondary plant metabolites exhibiting diverse properties. GABA accumulation is observed in stressed plants (20), and BABA treatment protects different plant species against various pathogens (21–23). Because the mode of action of BABA is still largely unknown in plants (41), we have analyzed the protective effect of this chemical in Arabidopsis. Our data indicate that, among the isomers tested, only BABA protects Arabidopsis against a virulent isolate of the oomycete pathogen P. parasitica. It therefore shows, as in other plant species (21–23), a high degree of specificity among aminobutyric acid isomers. Soil-drench treatment at a concentration as low as 8 mg/liter given 1 day before inoculation was sufficient to protect the plants. These results are consistent with protection observed in other plants (21, 22, 25). We have also shown that BABA is protecting Arabidopsis against pathogenic bacteria at a level similar to protection attributable to chemically induced resistance (35, 39). Hence, BABA protects different plant species against different fungi (21), a bacterium, a nematode (22), and a virus (23), demonstrating the broad range of activity of this chemical. Importantly, in vitro and in vivo experiments with diverse fungi and bacteria or P. parasitica show that BABA has no direct toxic effect. Furthermore, BABA is not metabolized in Arabidopsis, ruling out the involvement of a BABA metabolite acting as an antimicrobial compound in the plant. BABA does not show any curative effect, as it protects Arabidopsis against P. parasitica only when applied before inoculation. Taken together, BABA-mediated resistance is most likely based on the activation of host resistance mechanisms.
Microscopic analysis of the interaction between P. parasitica and BABA-treated Arabidopsis suggests that active defense mechanisms are involved in BABA-mediated resistance. Arabidopsis plants treated with BABA show typical responses observed in the course of induced resistance (35). Treatment with a high concentration of BABA leads to callose deposition termed papillae at almost all attempted penetration sites. At a lower concentration, a spectrum of responses from phenocopy HR to trailing necroses was observed, demonstrating a modulation of the plant response depending on the endogenous concentration of BABA. Importantly, BABA induced callose deposition only after attempted penetration of the epidermis by P. parasitica; spontaneous deposition without prior inoculation was never observed. Thus, BABA may induce resistance by accelerating the normal responses of the plant to infection, leading to a higher level of resistance. The same phenomenon was observed after infection with a virulent bacterium. In this case, soil-drench treatments with BABA did not induce PR-1 mRNA accumulation but conditioned the plant to induce this defense gene more rapidly after infection. Indeed, the plants reacted as fast as after infection with avirulent bacteria. Therefore, BABA enhances defense mechanisms triggered upon sensing of the pathogen by the plant, as no changes are detected in BABA-treated plants before infection. This phenomenon is known as potentiation or conditioning (41–43).
The observation that BABA induces resistance against P. parasitica in transgenic NahG plants suggests that BABA could activate the SAR pathway downstream of SA accumulation. However, the protection of npr1 mutants and the fact that BABA does not induce accumulation of PR-1 mRNA make this hypothesis unlikely. The plant hormones JA and ethylene have been implicated in a separate defense transduction pathway (10). Since BABA-induced resistance against P. parasitica is not dependent on sensitivity to JA and ethylene, this rules out the involvement of the resistance mechanisms mediated by these signaling molecules. Furthermore, the phytoalexin camalexin is also not a key factor of the BABA-induced resistance against P. parasitica, as camalexin-deficient mutants were fully protected (data not shown). Papillae are formed extensively after P. parasitica infection in BABA-treated Arabidopsis, a phenomenon rarely observed in nontreated controls. Therefore, this structural barrier could be sufficient to completely block P. parasitica penetration. After BABA treatment, even in the absence of PR protein accumulation, production of massive papillae is detected. Thus, the observed BABA-mediated conditioning leading to an earlier and stronger papilla formation may explain resistance against a normally virulent P. parasitica even in mutants impaired in the SAR transduction pathway. It demonstrates the value of papillae as an early defensive barrier sufficient to block P. parasitica penetration, making downstream defense mechanisms, such as PR proteins or camalexin, no longer necessary. This also suggests that BABA acts at a very early step in plant-pathogen interactions, probably at the recognition level. Interestingly, protection against bacteria is dependent of the SAR transduction pathway, as BABA potentiates PR-1 mRNA accumulation, and both transgenic NahG plants and npr1 mutants were not protected. These results are in agreement with data obtained in tobacco where BABA protects against the tobacco mosaic virus through an SA-dependent signal transduction pathway (23). Hence, different mechanisms of protection are effective against distinct pathogens, and BABA can stimulate the plant to deploy such pathogen-specific reactions much faster.
All of these observations highlight a new aspect of the biological action of the nonprotein amino acid BABA. Clearly, BABA enhances resistance through potentiation of pathogen–specific plant-defense responses, leading to a restriction of pathogen growth and spread. Furthermore, the observed early papilla formation obviously acts independently of known signaling cascades. These experiments add to our understanding of the importance of induced defense responses in plants. The site of action of BABA represents an attractive target for the development of novel crop protectants, which capitalize on the natural potential of plants to ward off pathogens.
Acknowledgments
We thank Drs. F. Mauch and T. Genoud for critical reading of the manuscript and helpful discussions; Drs. U. Gisi and E. Moesinger (Novartis, Basel) for the kind gift of 14C-labeled GABA and BABA; and G. Rigoli for excellent technical assistance. This work was supported by Swiss National Foundation Grants 3100-049279.96 (to B.M.M.) and 3100–055662.98 (to J.-P.M.) and Office Fédéral de l'Éducation et de la Science (OFES) Grant 96.0233 (to B.M.M. and J.-P.M.).
Abbreviations
- BABA
β-aminobutyric acid
- GABA
γ-aminobutyric acid
- HR
hypersensitive response
- PR
pathogenesis related
- SA
salicylic acid
- JA
jasmonic acid
- SAR
systemic acquired resistance
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.230416897.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.230416897
References
- 1.Van der Biezen E A, Jones J D. Trends Biochem Sci. 1998;23:454–456. doi: 10.1016/s0968-0004(98)01311-5. [DOI] [PubMed] [Google Scholar]
- 2.Richberg M H, Aviv D H, Dangl J L. Curr Opin Plant Biol. 1998;1:480–485. doi: 10.1016/s1369-5266(98)80039-3. [DOI] [PubMed] [Google Scholar]
- 3.Botella M A, Parker J E, Frost L N, Bittner-Eddy P D, Beynon J L, Daniels M J, Holub E B, Jones J D G. Plant Cell. 1998;10:1847–1860. doi: 10.1105/tpc.10.11.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Metraux J-P, Signer H, Ryals J, Ward E, Wyss-Benz M. Science. 1990;250:1004–1006. doi: 10.1126/science.250.4983.1004. [DOI] [PubMed] [Google Scholar]
- 5.Ward R E, Uknes S J, Williams S C, Dincher S S, Wiederhold D L, Alexander D C, Ahl-Goy P, Métraux J-P, Ryals J A. Plant Cell. 1991;3:1085–1094. doi: 10.1105/tpc.3.10.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Görlach J, Volrath S, Knauf-Beiter G, Hengy G, Beckhove U, Kogel K-H, Oostendorp M, Staub T, Ward E, Kessmann H, et al. Plant Cell. 1996;8:629–643. doi: 10.1105/tpc.8.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mur L A J, Naylor G, Warner S A J, Sugars J M, White R F, Draper J. Plant J. 1996;9:559–571. [Google Scholar]
- 8.Cao H, Bowling S A, Gordon A S, Dong X. Plant Cell. 1994;6:1583–1592. doi: 10.1105/tpc.6.11.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lawton K, Weymann K, Friedrich L, Vernooij B, Uknes S, Ryals J. Mol Plant Microbe Interact. 1995;8:863–870. doi: 10.1094/mpmi-8-0863. [DOI] [PubMed] [Google Scholar]
- 10.Thomma B P H J, Eggermont K, Penninckx I A M A, Mauch-Mani B, Vogelsang R, Cammue B P A, Broekaert W F. Proc Natl Acad Sci USA. 1998;95:15107–15111. doi: 10.1073/pnas.95.25.15107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Penninckx I A M A, Eggermont K, Terras F R, Thomma B P, De Samblanx G W, Buchala A, Métraux J-P, Manners J M, Broeckaert W F. Plant Cell. 1996;8:2309–2323. doi: 10.1105/tpc.8.12.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomma B P H J, Eggermont K, Tierens K F M-J, Broeckaert W F. Plant Physiol. 1999;121:1093–1101. doi: 10.1104/pp.121.4.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bent A F, Innes R W, Ecker J R, Staskawicz B J. Mol Plant Microbe Interact. 1992;5:372–378. doi: 10.1094/mpmi-5-372. [DOI] [PubMed] [Google Scholar]
- 14.Staswick P E, Su W P, Howell S H. Proc Natl Acad Sci USA. 1992;89:6837–6840. doi: 10.1073/pnas.89.15.6837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bleecker A B, Estelle M A, Somerville C, Kende H. Science. 1988;241:1086–1089. doi: 10.1126/science.241.4869.1086. [DOI] [PubMed] [Google Scholar]
- 16.Staswick P E, Yuen G Y, Lehman C C. Plant J. 1998;15:747–754. doi: 10.1046/j.1365-313x.1998.00265.x. [DOI] [PubMed] [Google Scholar]
- 17.Knoester M, van Loon L C, van den Heuvel J, Hennig J, Linthorst H J M. Proc Natl Acad Sci USA. 1998;95:1933–1937. doi: 10.1073/pnas.95.4.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waagepetersen H S, Sonnewald U, Schousboe A. J Neurochem. 1999;73:1335–1342. doi: 10.1046/j.1471-4159.1999.0731335.x. [DOI] [PubMed] [Google Scholar]
- 19.Schmieden V, Betz H. Mol Pharmacol. 1995;48:919–927. [PubMed] [Google Scholar]
- 20.Shelp B J, Bown A W, McLean M D. Trends Plant Sci. 1999;4:446–452. doi: 10.1016/s1360-1385(99)01486-7. [DOI] [PubMed] [Google Scholar]
- 21.Cohen Y. Phytopathology. 1994;84:55–59. [Google Scholar]
- 22.Oka Y, Cohen Y, Spiegel Y. Phytopathology. 1999;89:1138–1143. doi: 10.1094/PHYTO.1999.89.12.1138. [DOI] [PubMed] [Google Scholar]
- 23.Siegrist J, Orober M, Buchenauer H. Physiol Mol Plant Pathol. 2000;56:95–106. [Google Scholar]
- 24.Cohen Y, Niderman T, Mösinger E, Fluhr R. Plant Physiol. 1994;104:59–66. doi: 10.1104/pp.104.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen Y. Physiol Mol Plant Pathol. 1994;44:273–288. [Google Scholar]
- 26.Mauch-Mani B, Slusarenko A J. Mol Plant Microbe Interact. 1994;7:378–383. [Google Scholar]
- 27.Wahlen M C, Innes R W, Bent A F, Staskawicz B J. Plant Cell. 1991;3:49–59. doi: 10.1105/tpc.3.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.King E O, Ward M K, Raney D E. J Lab Clin Med. 1954;44:301–307. [PubMed] [Google Scholar]
- 29.Hooykaas P J J, Roobol C, Schilperoort R A. J Gen Microbiol. 1979;110:99–109. [Google Scholar]
- 30.Smith M M, McCully M E. Protoplasma. 1978;95:229–254. [Google Scholar]
- 31.Murashige T, Skoog F. Plant Physiol. 1962;15:473–497. [Google Scholar]
- 32.Altman T, Damm B, Halfter U, Willmitzer L, Moris P C. In: Methods in Arabidopsis Research. Koncz C, Chua N-H, Schell J, editors. Singapore: World Scientific; 1992. pp. 310–330. [Google Scholar]
- 33.Larkin P J. Planta. 1976;128:213–216. doi: 10.1007/BF00393231. [DOI] [PubMed] [Google Scholar]
- 34.Chirjwin J M, Przybyla A E, McDonald R J, Rutter W J. Biochemistry. 1979;18:5295–5299. [Google Scholar]
- 35.Uknes S, Mauch-Mani B, Moyer M, Potter S, Williams S, Dincher S, Chandler D, Slusarenko A, Ward E, Ryals J. Plant Cell. 1992;4:645–656. doi: 10.1105/tpc.4.6.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sticher L, Mauch-Mani B, Metraux J-P. Annu Rev Phytopathol. 1997;35:235–270. doi: 10.1146/annurev.phyto.35.1.235. [DOI] [PubMed] [Google Scholar]
- 37.Parker J E, Szabo V, Staskawicz B J, Lister C, Dean C, Daniels M J, Jones J D G. Plant J. 1993;4:821–831. [Google Scholar]
- 38.Aist J R. Annu Rev Phytopathol. 1976;14:145–163. [Google Scholar]
- 39.Lawton K A, Friederich L, Hunt M, Weymann K, Delaney T, Kessmann H, Staub T, Ryals J. Plant J. 1996;10:71–82. doi: 10.1046/j.1365-313x.1996.10010071.x. [DOI] [PubMed] [Google Scholar]
- 40.Epple P, Apel K, Bohlmann H. Plant Physiol. 1995;109:813–820. doi: 10.1104/pp.109.3.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kessmann H, Staub T, Hofmann C, Maetzke T, Herzog J, Ward E, Uknes S, Ryals J. Annu Rev Phytopathol. 1994;32:439–459. doi: 10.1146/annurev.py.32.090194.002255. [DOI] [PubMed] [Google Scholar]
- 42.Kauss H, Franke R, Krause K, Conrath U, Jeblick W, Grimmig B, Matern U. Plant Physiol. 1993;102:459–466. doi: 10.1104/pp.102.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Katz V A, Thulke O U, Conrath U. Plant Physiol. 1998;117:1333–1339. doi: 10.1104/pp.117.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rohringer R, Kim W K, Samborski D J, Howes N K. Phytopathology. 1977;67:808–810. [Google Scholar]
- 45.Keogh R C, Deverall B J, McLeod S. Trans Br Mycol Soc. 1980;74:329–333. [Google Scholar]