Abstract
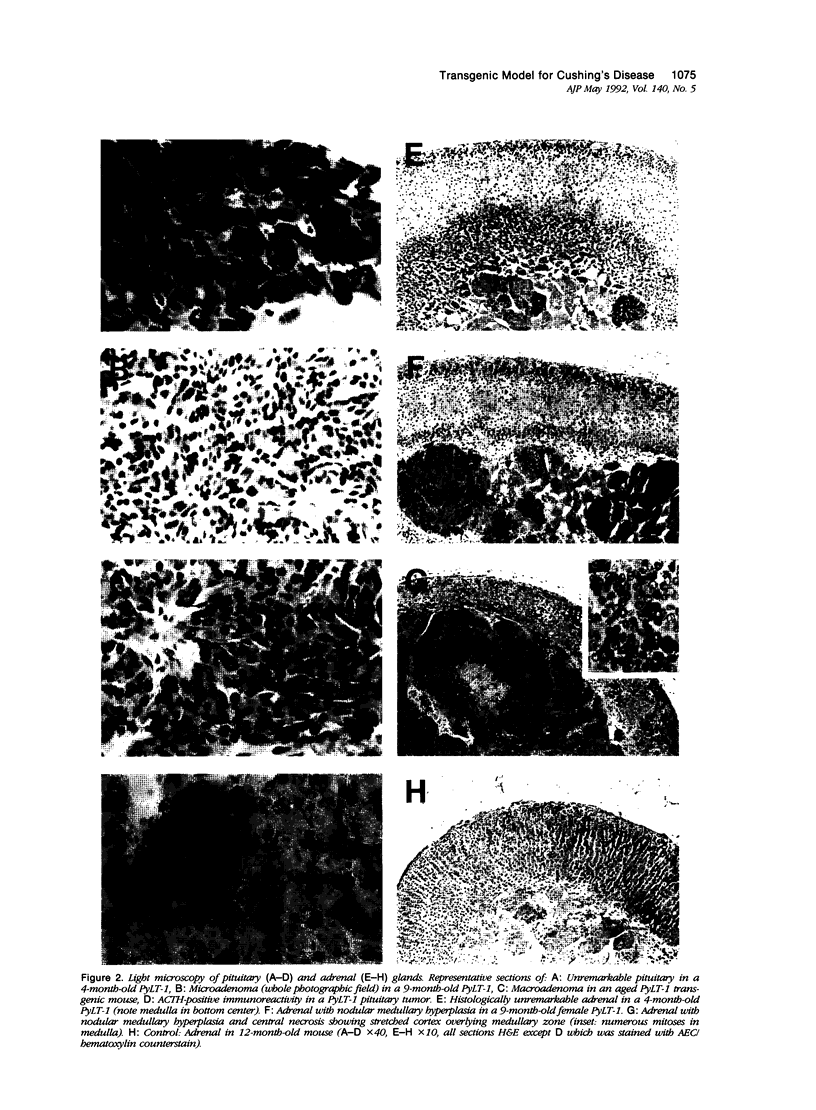
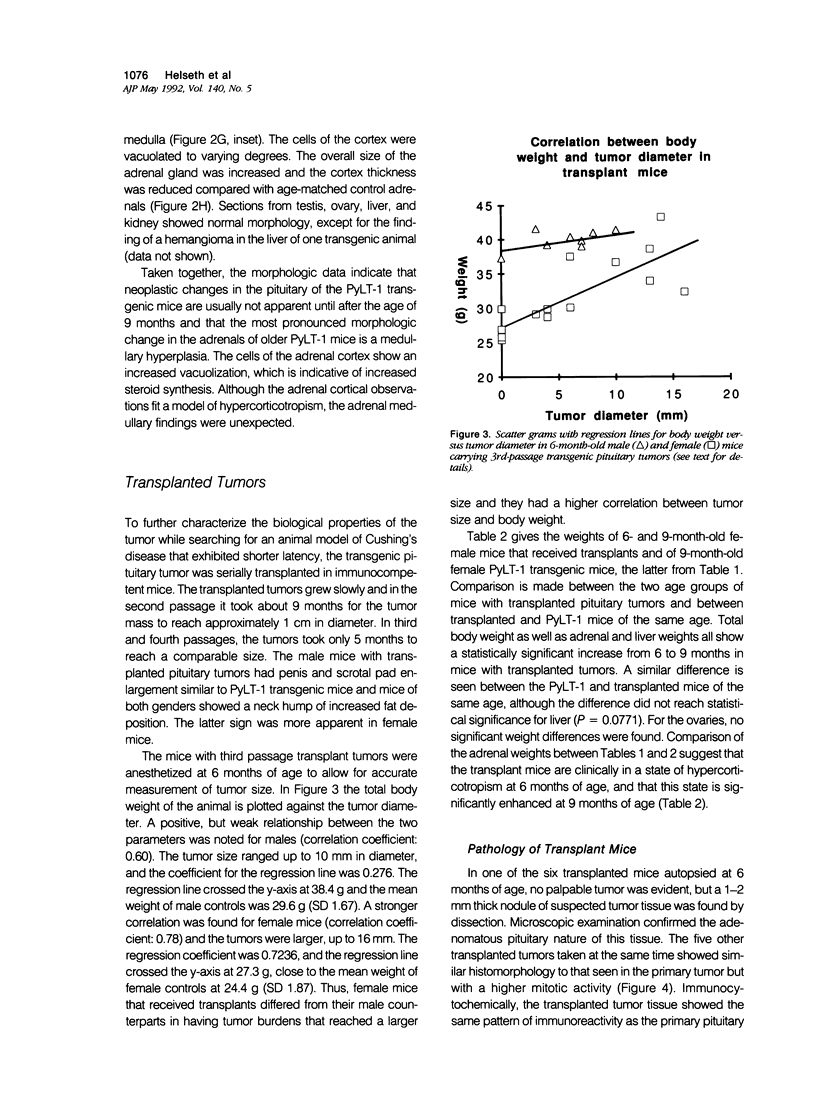
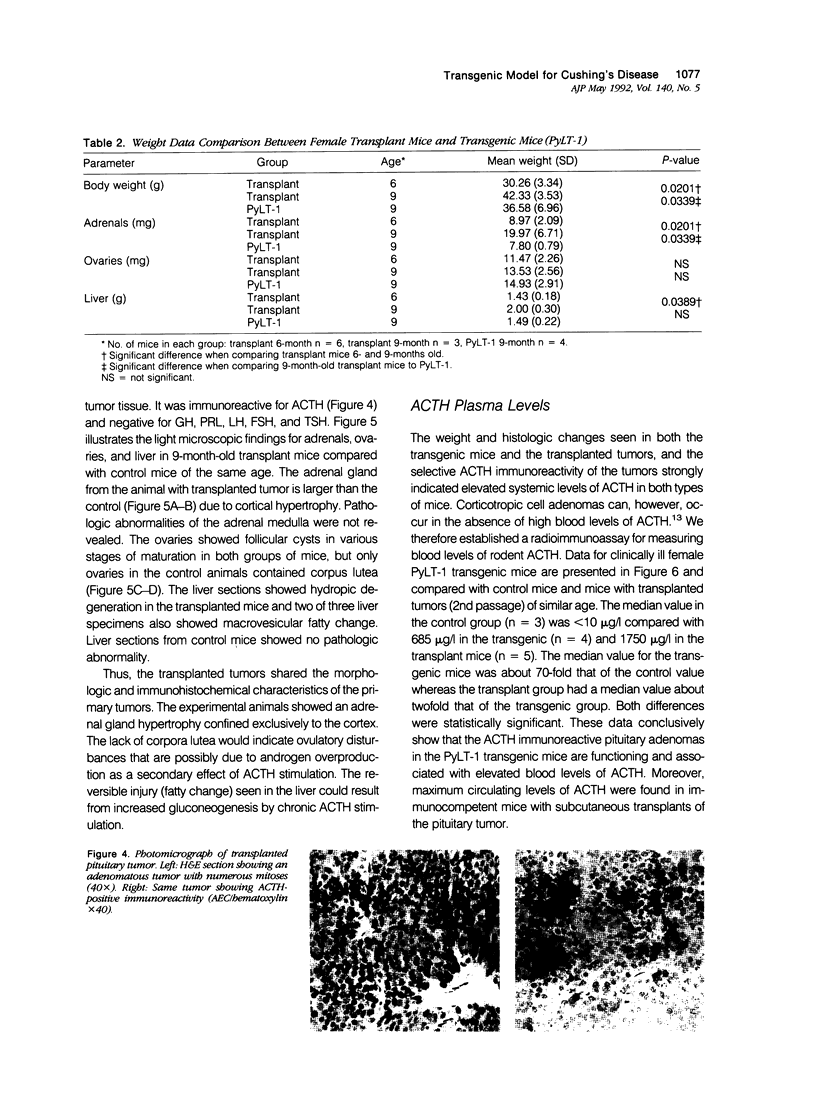
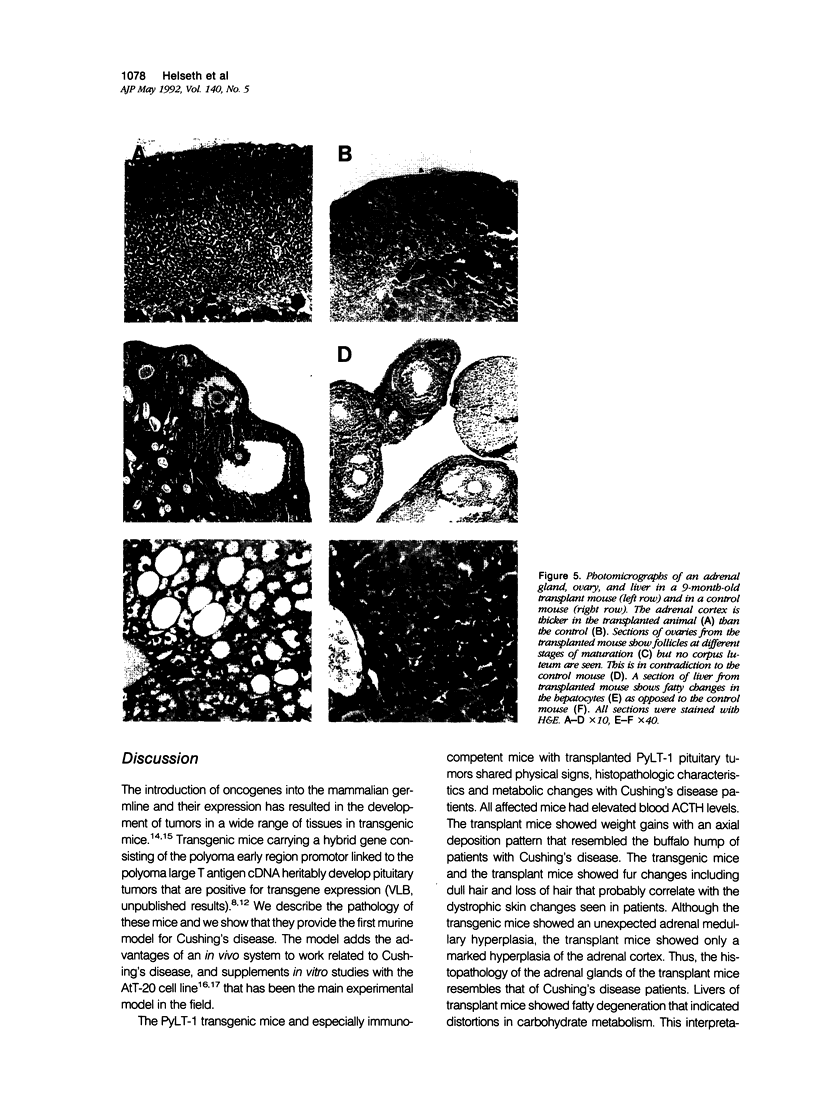
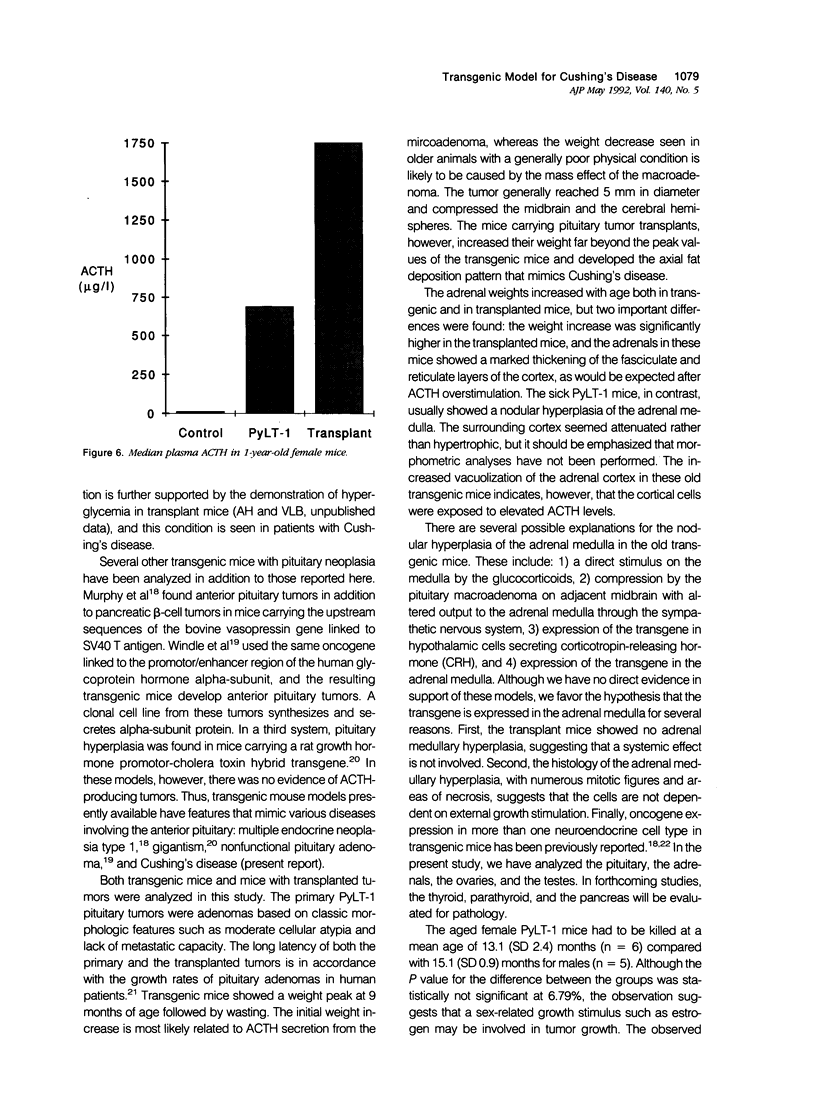
Transgenic mice that developed adrenocorticotropic hormone (ACTH)-producing pituitary tumors were generated with the polyoma early region promotor linked to a cDNA encoding polyoma large T antigen (PyLT). Light microscopic examination of the pituitaries showed normal morphology at 4 months of age, either unremarkable morphology or microadenoma formation at 9 months of age, and up to 5 mm large adenomas in clinically ill transgenic mice at 13-16 months of age. At age 9 months, transgenic mice weighed significantly more than corresponding control mice, but they began wasting at approximately 1 year of age. The adrenal glands of these older PyLT-1 mice showed a weight increase and exhibited a medullary hyperplasia. Subcutaneous transplants of transgenic pituitary tumors to nontransgenic, immunocompetent mice resulted in tumors with a morphology and ACTH immunoreactivity similar to the primary tumor. The effects of hypercorticotropism were more enhanced and occurred with a shorter latency in the mice carrying transgene pituitary transplants than in the PyLT-1 transgenic mice themselves. Moreover, these transplanted mice showed a weight increase with an axial deposition pattern and hypertrophy of the adrenal cortex that resembled the findings in human Cushing's disease. Plasma ACTH levels were significantly increased in clinically ill transgenic mice and even higher levels were found in the transplant mice. Thus, both murine models should be useful for studying Cushing's disease.
Full text
PDF


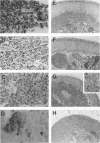
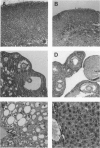
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aron D. C. Cushing's syndrome: current concepts in diagnosis and treatment. Compr Ther. 1987 Dec;13(12):37–44. [PubMed] [Google Scholar]
- Bautch V. L. Effects of polyoma virus oncogenes in transgenic mice. Mol Biol Med. 1989 Aug;6(4):309–317. [PubMed] [Google Scholar]
- Bautch V. L., Toda S., Hassell J. A., Hanahan D. Endothelial cell tumors develop in transgenic mice carrying polyoma virus middle T oncogene. Cell. 1987 Nov 20;51(4):529–537. doi: 10.1016/0092-8674(87)90122-x. [DOI] [PubMed] [Google Scholar]
- Burton F. H., Hasel K. W., Bloom F. E., Sutcliffe J. G. Pituitary hyperplasia and gigantism in mice caused by a cholera toxin transgene. Nature. 1991 Mar 7;350(6313):74–77. doi: 10.1038/350074a0. [DOI] [PubMed] [Google Scholar]
- Ciric I., Mikhael M., Stafford T., Lawson L., Garces R. Transsphenoidal microsurgery of pituitary macroadenomas with long-term follow-up results. J Neurosurg. 1983 Sep;59(3):395–401. doi: 10.3171/jns.1983.59.3.0395. [DOI] [PubMed] [Google Scholar]
- Cory S., Adams J. M. Transgenic mice and oncogenesis. Annu Rev Immunol. 1988;6:25–48. doi: 10.1146/annurev.iy.06.040188.000325. [DOI] [PubMed] [Google Scholar]
- Drouin J., Charron J., Gagner J. P., Jeannotte L., Nemer M., Plante R. K., Wrange O. Pro-opiomelanocortin gene: a model for negative regulation of transcription by glucocorticoids. J Cell Biochem. 1987 Dec;35(4):293–304. doi: 10.1002/jcb.240350404. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Dissecting multistep tumorigenesis in transgenic mice. Annu Rev Genet. 1988;22:479–519. doi: 10.1146/annurev.ge.22.120188.002403. [DOI] [PubMed] [Google Scholar]
- Klibanski A., Zervas N. T. Diagnosis and management of hormone-secreting pituitary adenomas. N Engl J Med. 1991 Mar 21;324(12):822–831. doi: 10.1056/NEJM199103213241207. [DOI] [PubMed] [Google Scholar]
- Loeffel S. C., Gillespie G. Y., Mirmiran S. A., Miller E. W., Golden P., Askin F. B., Siegal G. P. Cellular immunolocalization of S100 protein within fixed tissue sections by monoclonal antibodies. Arch Pathol Lab Med. 1985 Feb;109(2):117–122. [PubMed] [Google Scholar]
- Murphy D., Bishop A., Rindi G., Murphy M. N., Stamp G. W., Hanson J., Polak J. M., Hogan B. Mice transgenic for a vasopressin-SV40 hybrid oncogene develop tumors of the endocrine pancreas and the anterior pituitary. A possible model for human multiple endocrine neoplasia type 1. Am J Pathol. 1987 Dec;129(3):552–566. [PMC free article] [PubMed] [Google Scholar]
- Nakamura M., Nakanishi S., Sueoka S., Imura H., Numa S. Effects of steroid hormones on the level of corticotropin messenger RNA activity in cultured mouse-pituitary-tumor cells. Eur J Biochem. 1978 May;86(1):61–66. doi: 10.1111/j.1432-1033.1978.tb12284.x. [DOI] [PubMed] [Google Scholar]
- Nicholson W. E., Davis D. R., Sherrell B. J., Orth D. N. Rapid radioimmunoassay for corticotropin in unextracted human plasma. Clin Chem. 1984 Feb;30(2):259–265. [PubMed] [Google Scholar]
- Oldfield E. H., Doppman J. L., Nieman L. K., Chrousos G. P., Miller D. L., Katz D. A., Cutler G. B., Jr, Loriaux D. L. Petrosal sinus sampling with and without corticotropin-releasing hormone for the differential diagnosis of Cushing's syndrome. N Engl J Med. 1991 Sep 26;325(13):897–905. doi: 10.1056/NEJM199109263251301. [DOI] [PubMed] [Google Scholar]
- Rindi G., Bishop A. E., Murphy D., Solcia E., Hogan B., Polak J. M. A morphological analysis endocrine tumour genesis in pancreas and anterior pituitary of AVP/SV40 transgenic mice. Virchows Arch A Pathol Anat Histopathol. 1988;412(3):255–266. doi: 10.1007/BF00737150. [DOI] [PubMed] [Google Scholar]
- Vale W., Spiess J., Rivier C., Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981 Sep 18;213(4514):1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Waterman M. R., Simpson E. R. Regulation of the biosynthesis of cytochromes P-450 involved in steroid hormone synthesis. Mol Cell Endocrinol. 1985 Feb;39(2):81–89. doi: 10.1016/0303-7207(85)90123-6. [DOI] [PubMed] [Google Scholar]
- Wick M. R., Siegal G. P., Mills S. E., Thompson R. C., Sawhney D., Fechner R. E. Dedifferentiated chondrosarcoma of bone. An immunohistochemical and lectin-histochemical study. Virchows Arch A Pathol Anat Histopathol. 1987;411(1):23–32. doi: 10.1007/BF00734510. [DOI] [PubMed] [Google Scholar]
- Windle J. J., Weiner R. I., Mellon P. L. Cell lines of the pituitary gonadotrope lineage derived by targeted oncogenesis in transgenic mice. Mol Endocrinol. 1990 Apr;4(4):597–603. doi: 10.1210/mend-4-4-597. [DOI] [PubMed] [Google Scholar]