Abstract
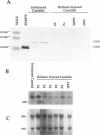
The localization and synthesis of basic fibroblast growth factor (bFGF) in the rat carotid artery were investigated at times of chronic smooth muscle cell proliferation. Immunocytochemical staining showed the presence of bFGF in the uninjured arterial wall, and after balloon injury, this cellular staining was decreased. Western and northern blot analyses likewise showed that the amount of bFGF protein and mRNA decreased after injury. A neutralizing antibody to bFGF was administered 4 and 5 days after injury and was found to have no effect on intimal smooth muscle cell proliferation. These data suggest that an increase in the expression of bFGF is not necessary for chronic smooth muscle cell proliferation observed after balloon catheter injury and that bFGF is not the major mitogen responsible for intimal smooth muscle cell proliferation.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brigstock D. R., Klagsbrun M., Sasse J., Farber P. A., Iberg N. Species-specific high molecular weight forms of basic fibroblast growth factor. Growth Factors. 1990;4(1):45–52. doi: 10.3109/08977199009011009. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clowes A. W., Reidy M. A., Clowes M. M. Kinetics of cellular proliferation after arterial injury. I. Smooth muscle growth in the absence of endothelium. Lab Invest. 1983 Sep;49(3):327–333. [PubMed] [Google Scholar]
- Clowes A. W., Reidy M. A., Clowes M. M. Mechanisms of stenosis after arterial injury. Lab Invest. 1983 Aug;49(2):208–215. [PubMed] [Google Scholar]
- Cordon-Cardo C., Vlodavsky I., Haimovitz-Friedman A., Hicklin D., Fuks Z. Expression of basic fibroblast growth factor in normal human tissues. Lab Invest. 1990 Dec;63(6):832–840. [PubMed] [Google Scholar]
- Fingerle J., Johnson R., Clowes A. W., Majesky M. W., Reidy M. A. Role of platelets in smooth muscle cell proliferation and migration after vascular injury in rat carotid artery. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8412–8416. doi: 10.1073/pnas.86.21.8412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florkiewicz R. Z., Baird A., Gonzalez A. M. Multiple forms of bFGF: differential nuclear and cell surface localization. Growth Factors. 1991;4(4):265–275. doi: 10.3109/08977199109043912. [DOI] [PubMed] [Google Scholar]
- Gajdusek C. M., Carbon S. Injury-induced release of basic fibroblast growth factor from bovine aortic endothelium. J Cell Physiol. 1989 Jun;139(3):570–579. doi: 10.1002/jcp.1041390317. [DOI] [PubMed] [Google Scholar]
- Gonzalez A. M., Buscaglia M., Ong M., Baird A. Distribution of basic fibroblast growth factor in the 18-day rat fetus: localization in the basement membranes of diverse tissues. J Cell Biol. 1990 Mar;110(3):753–765. doi: 10.1083/jcb.110.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D., Ferrara N., Haaparanta T., Neufeld G. Basic fibroblast growth factor: expression in cultured bovine vascular smooth muscle cells. Eur J Cell Biol. 1988 Apr;46(1):144–151. [PubMed] [Google Scholar]
- Lindner V., Lappi D. A., Baird A., Majack R. A., Reidy M. A. Role of basic fibroblast growth factor in vascular lesion formation. Circ Res. 1991 Jan;68(1):106–113. doi: 10.1161/01.res.68.1.106. [DOI] [PubMed] [Google Scholar]
- Lindner V., Reidy M. A. Proliferation of smooth muscle cells after vascular injury is inhibited by an antibody against basic fibroblast growth factor. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3739–3743. doi: 10.1073/pnas.88.9.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majack R. A., Majesky M. W., Goodman L. V. Role of PDGF-A expression in the control of vascular smooth muscle cell growth by transforming growth factor-beta. J Cell Biol. 1990 Jul;111(1):239–247. doi: 10.1083/jcb.111.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majesky M. W., Benditt E. P., Schwartz S. M. Expression and developmental control of platelet-derived growth factor A-chain and B-chain/Sis genes in rat aortic smooth muscle cells. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1524–1528. doi: 10.1073/pnas.85.5.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majesky M. W., Reidy M. A., Bowen-Pope D. F., Hart C. E., Wilcox J. N., Schwartz S. M. PDGF ligand and receptor gene expression during repair of arterial injury. J Cell Biol. 1990 Nov;111(5 Pt 1):2149–2158. doi: 10.1083/jcb.111.5.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil P. L., Muthukrishnan L., Warder E., D'Amore P. A. Growth factors are released by mechanically wounded endothelial cells. J Cell Biol. 1989 Aug;109(2):811–822. doi: 10.1083/jcb.109.2.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell P. P., Klagsbrun M. Three forms of rat basic fibroblast growth factor are made from a single mRNA and localize to the nucleus. J Cell Physiol. 1991 Aug;148(2):202–210. doi: 10.1002/jcp.1041480204. [DOI] [PubMed] [Google Scholar]
- Prats H., Kaghad M., Prats A. C., Klagsbrun M., Lélias J. M., Liauzun P., Chalon P., Tauber J. P., Amalric F., Smith J. A. High molecular mass forms of basic fibroblast growth factor are initiated by alternative CUG codons. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1836–1840. doi: 10.1073/pnas.86.6.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reidy M. A., Schwartz S. M. Endothelial regeneration. III. Time course of intimal changes after small defined injury to rat aortic endothelium. Lab Invest. 1981 Apr;44(4):301–308. [PubMed] [Google Scholar]
- Renko M., Quarto N., Morimoto T., Rifkin D. B. Nuclear and cytoplasmic localization of different basic fibroblast growth factor species. J Cell Physiol. 1990 Jul;144(1):108–114. doi: 10.1002/jcp.1041440114. [DOI] [PubMed] [Google Scholar]
- Saksela O., Rifkin D. B. Release of basic fibroblast growth factor-heparan sulfate complexes from endothelial cells by plasminogen activator-mediated proteolytic activity. J Cell Biol. 1990 Mar;110(3):767–775. doi: 10.1083/jcb.110.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlodavsky I., Folkman J., Sullivan R., Fridman R., Ishai-Michaeli R., Sasse J., Klagsbrun M. Endothelial cell-derived basic fibroblast growth factor: synthesis and deposition into subendothelial extracellular matrix. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2292–2296. doi: 10.1073/pnas.84.8.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlodavsky I., Fuks Z., Ishai-Michaeli R., Bashkin P., Levi E., Korner G., Bar-Shavit R., Klagsbrun M. Extracellular matrix-resident basic fibroblast growth factor: implication for the control of angiogenesis. J Cell Biochem. 1991 Feb;45(2):167–176. doi: 10.1002/jcb.240450208. [DOI] [PubMed] [Google Scholar]
- Weich H. A., Iberg N., Klagsbrun M., Folkman J. Expression of acidic and basic fibroblast growth factors in human and bovine vascular smooth muscle cells. Growth Factors. 1990;2(4):313–320. doi: 10.3109/08977199009167026. [DOI] [PubMed] [Google Scholar]
- Weiner H. L., Swain J. L. Acidic fibroblast growth factor mRNA is expressed by cardiac myocytes in culture and the protein is localized to the extracellular matrix. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2683–2687. doi: 10.1073/pnas.86.8.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]