Abstract
Rapid expression of mRNA encoding vascular cell adhesion molecule-1 (VCAM-1) was induced by tumor necrosis factor (TNF) in fibroblast-like cells obtained from synovial tissue. Both alternatively spliced forms of VCAM-1 mRNA were detected by polymerase chain reaction in TNF-stimulated fibroblast-like synoviocytes. Western blotting analysis showed that two distinct proteins, reactive with an anti-VCAM-1 anti-sera, were expressed by 2 hours of TNF stimulation in both synoviocytes and human umbilical cord vein endothelial cells (HUVEC). The majority of HUVEC and synoviocytes displayed VCAM-1 surface expression after several hours of TNF stimulation. In contrast, dermal fibroblasts upregulated intercellular adhesion molecule-1 (ICAM-1) but not VCAM-1 expression in response to TNF. These results indicate that VCAM-1 and ICAM-1 expression can be differentially regulated and suggest tissue specific regulation of VCAM-1 expression. Furthermore, these findings may provide an explanation for the chronic retention and activation of long-lived lymphocytes and monocytes, which express VLA-4 (the receptor for VCAM-1), in the synovium in rheumatoid arthritis.
Full text
PDF

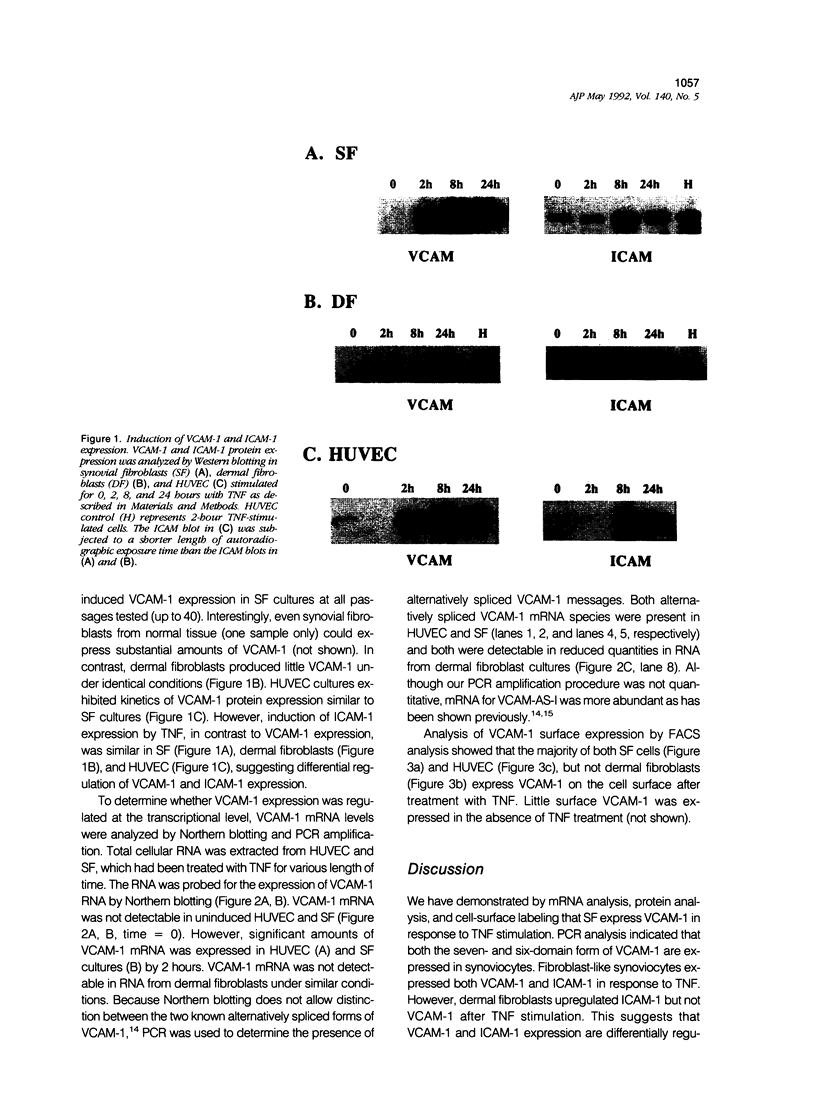


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahamsen T. G., Johnson P. M., Natvig J. B. Membrane characteristics of adherent cells dissociated from rheumatoid synovial tissue. Clin Exp Immunol. 1977 Jun;28(3):474–483. [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Chu C. Q., Field M., Feldmann M., Maini R. N. Localization of tumor necrosis factor alpha in synovial tissues and at the cartilage-pannus junction in patients with rheumatoid arthritis. Arthritis Rheum. 1991 Sep;34(9):1125–1132. doi: 10.1002/art.1780340908. [DOI] [PubMed] [Google Scholar]
- Cybulsky M. I., Fries J. W., Williams A. J., Sultan P., Davis V. M., Gimbrone M. A., Jr, Collins T. Alternative splicing of human VCAM-1 in activated vascular endothelium. Am J Pathol. 1991 Apr;138(4):815–820. [PMC free article] [PubMed] [Google Scholar]
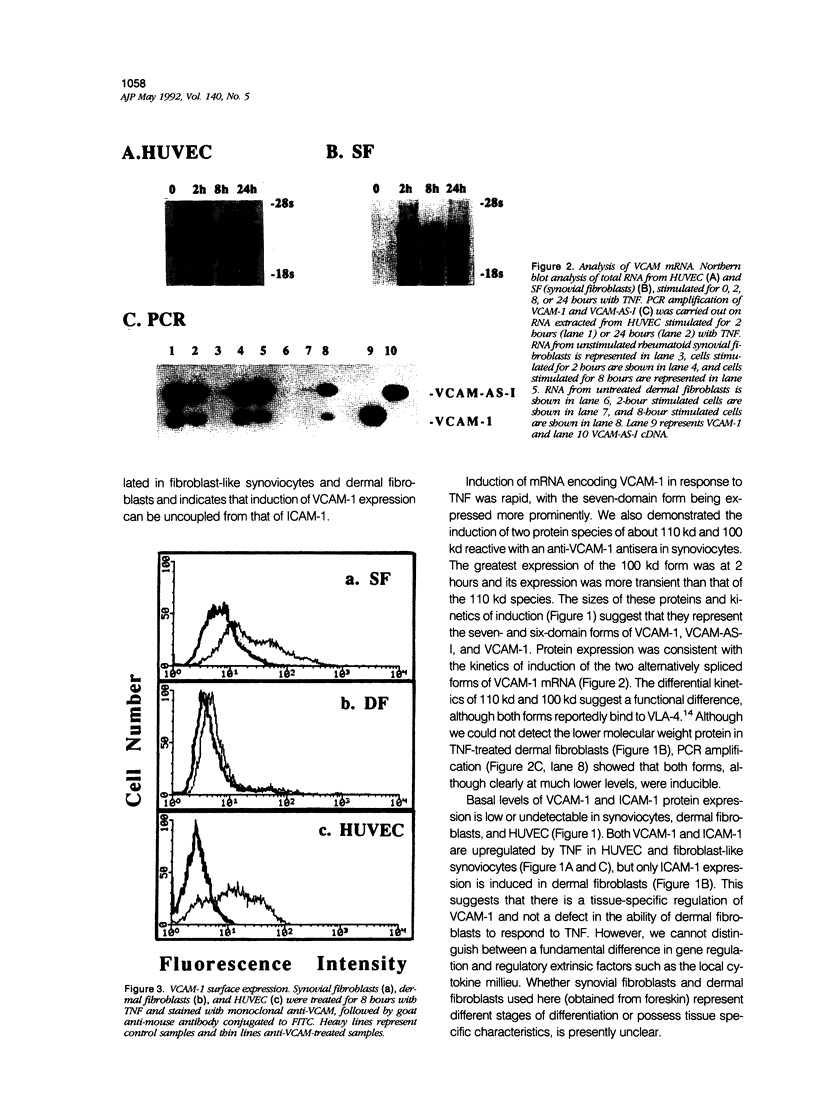
- Damle N. K., Aruffo A. Vascular cell adhesion molecule 1 induces T-cell antigen receptor-dependent activation of CD4+T lymphocytes. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6403–6407. doi: 10.1073/pnas.88.15.6403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elices M. J., Osborn L., Takada Y., Crouse C., Luhowskyj S., Hemler M. E., Lobb R. R. VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA-4/fibronectin binding site. Cell. 1990 Feb 23;60(4):577–584. doi: 10.1016/0092-8674(90)90661-w. [DOI] [PubMed] [Google Scholar]
- Goldring S. R., Mahaffey J. E., Rosenblatt M., Dayer J. M., Potts J. T., Jr, Krane S. M. Parathyroid hormone inhibitors: comparison of biological activity in bone- and skin-derived tissue. J Clin Endocrinol Metab. 1979 Apr;48(4):655–659. doi: 10.1210/jcem-48-4-655. [DOI] [PubMed] [Google Scholar]
- Goldring S. R., Stephenson M. L., Downie E., Krane S. M., Korn J. H. Heterogeneity in hormone responses and patterns of collagen synthesis in cloned dermal fibroblasts. J Clin Invest. 1990 Mar;85(3):798–803. doi: 10.1172/JCI114506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve J. M., Davis G., Meyer A. M., Forte C. P., Yost S. C., Marlor C. W., Kamarck M. E., McClelland A. The major human rhinovirus receptor is ICAM-1. Cell. 1989 Mar 10;56(5):839–847. doi: 10.1016/0092-8674(89)90688-0. [DOI] [PubMed] [Google Scholar]
- Hemler M. E., Huang C., Takada Y., Schwarz L., Strominger J. L., Clabby M. L. Characterization of the cell surface heterodimer VLA-4 and related peptides. J Biol Chem. 1987 Aug 25;262(24):11478–11485. [PubMed] [Google Scholar]
- Hemler M. E. VLA proteins in the integrin family: structures, functions, and their role on leukocytes. Annu Rev Immunol. 1990;8:365–400. doi: 10.1146/annurev.iy.08.040190.002053. [DOI] [PubMed] [Google Scholar]
- Hession C., Tizard R., Vassallo C., Schiffer S. B., Goff D., Moy P., Chi-Rosso G., Luhowskyj S., Lobb R., Osborn L. Cloning of an alternate form of vascular cell adhesion molecule-1 (VCAM1). J Biol Chem. 1991 Apr 15;266(11):6682–6685. [PubMed] [Google Scholar]
- Iguchi T., Kurosaka M., Ziff M. Electron microscopic study of HLA-DR and monocyte/macrophage staining cells in the rheumatoid synovial membrane. Arthritis Rheum. 1986 May;29(5):600–613. doi: 10.1002/art.1780290504. [DOI] [PubMed] [Google Scholar]
- Klareskog L., Forsum U., Scheynius A., Kabelitz D., Wigzell H. Evidence in support of a self-perpetuating HLA-DR-dependent delayed-type cell reaction in rheumatoid arthritis. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3632–3636. doi: 10.1073/pnas.79.11.3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi I., Ziff M. Electron microscopic studies of lymphoid cells in the rheumatoid synovial membrane. Arthritis Rheum. 1973 Jul-Aug;16(4):471–486. doi: 10.1002/art.1780160407. [DOI] [PubMed] [Google Scholar]
- Koch A. E., Burrows J. C., Haines G. K., Carlos T. M., Harlan J. M., Leibovich S. J. Immunolocalization of endothelial and leukocyte adhesion molecules in human rheumatoid and osteoarthritic synovial tissues. Lab Invest. 1991 Mar;64(3):313–320. [PubMed] [Google Scholar]
- Krane S. M. Heberden Oration 1980: aspects of the cell biology of the rheumatoid synovial lesion. Ann Rheum Dis. 1981 Oct;40(5):433–448. doi: 10.1136/ard.40.5.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krane S. M., Simon L. S. Rheumatoid arthritis: clinical features and pathogenetic mechanisms. Med Clin North Am. 1986 Mar;70(2):263–284. doi: 10.1016/s0025-7125(16)30953-1. [DOI] [PubMed] [Google Scholar]
- Osborn L., Hession C., Tizard R., Vassallo C., Luhowskyj S., Chi-Rosso G., Lobb R. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell. 1989 Dec 22;59(6):1203–1211. doi: 10.1016/0092-8674(89)90775-7. [DOI] [PubMed] [Google Scholar]
- Poulter L. W., Duke O., Hobbs S., Janossy G., Panayi G., Seymour G. The involvement of interdigitating (antigen-presenting) cells in the pathogenesis of rheumatoid arthritis. Clin Exp Immunol. 1983 Feb;51(2):247–254. [PMC free article] [PubMed] [Google Scholar]
- Rice G. E., Bevilacqua M. P. An inducible endothelial cell surface glycoprotein mediates melanoma adhesion. Science. 1989 Dec 8;246(4935):1303–1306. doi: 10.1126/science.2588007. [DOI] [PubMed] [Google Scholar]
- Rice G. E., Munro J. M., Bevilacqua M. P. Inducible cell adhesion molecule 110 (INCAM-110) is an endothelial receptor for lymphocytes. A CD11/CD18-independent adhesion mechanism. J Exp Med. 1990 Apr 1;171(4):1369–1374. doi: 10.1084/jem.171.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice G. E., Munro J. M., Corless C., Bevilacqua M. P. Vascular and nonvascular expression of INCAM-110. A target for mononuclear leukocyte adhesion in normal and inflamed human tissues. Am J Pathol. 1991 Feb;138(2):385–393. [PMC free article] [PubMed] [Google Scholar]
- Rothman B. L., Blue M. L., Kelley K. A., Wunderlich D., Mierz D. V., Aune T. M. Human T cell activation by OKT3 is inhibited by a monoclonal antibody to CD44. J Immunol. 1991 Oct 15;147(8):2493–2499. [PubMed] [Google Scholar]
- Voyta J. C., Via D. P., Butterfield C. E., Zetter B. R. Identification and isolation of endothelial cells based on their increased uptake of acetylated-low density lipoprotein. J Cell Biol. 1984 Dec;99(6):2034–2040. doi: 10.1083/jcb.99.6.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Seventer G. A., Newman W., Shimizu Y., Nutman T. B., Tanaka Y., Horgan K. J., Gopal T. V., Ennis E., O'Sullivan D., Grey H. Analysis of T cell stimulation by superantigen plus major histocompatibility complex class II molecules or by CD3 monoclonal antibody: costimulation by purified adhesion ligands VCAM-1, ICAM-1, but not ELAM-1. J Exp Med. 1991 Oct 1;174(4):901–913. doi: 10.1084/jem.174.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]