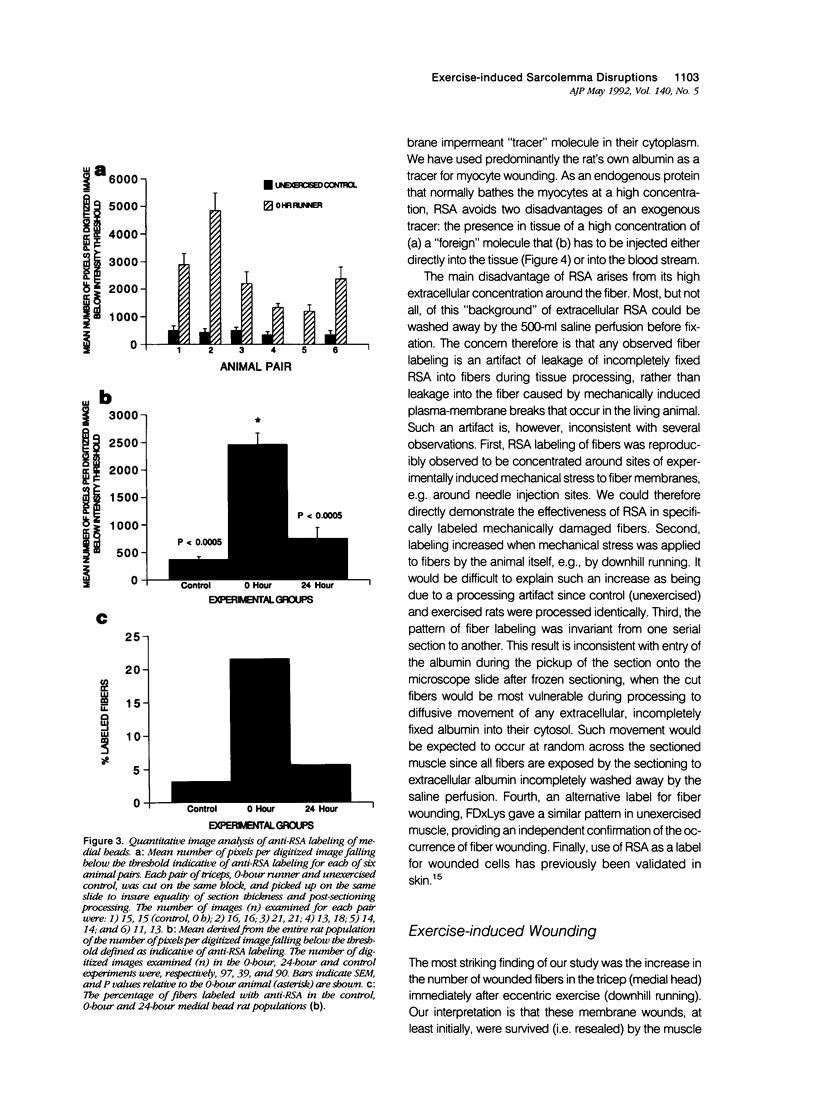
Abstract
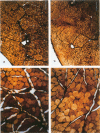
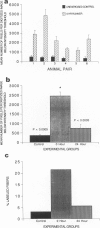
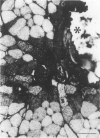
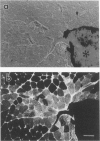
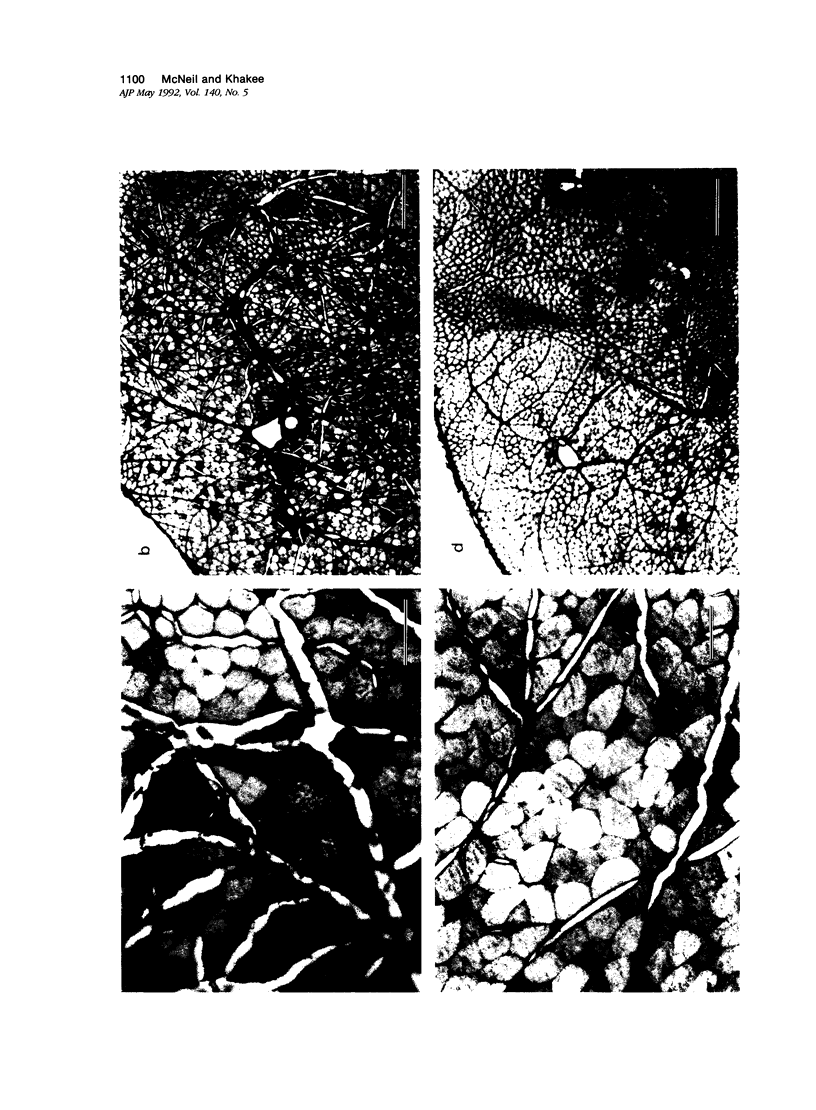
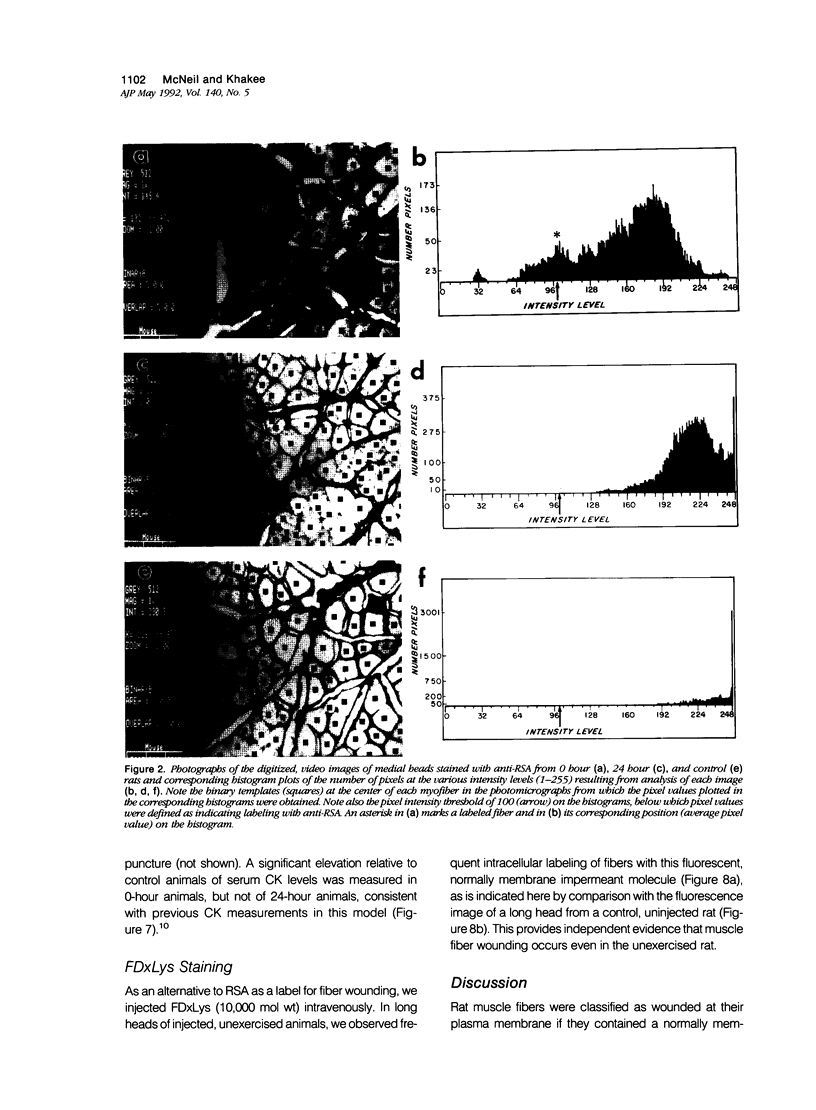
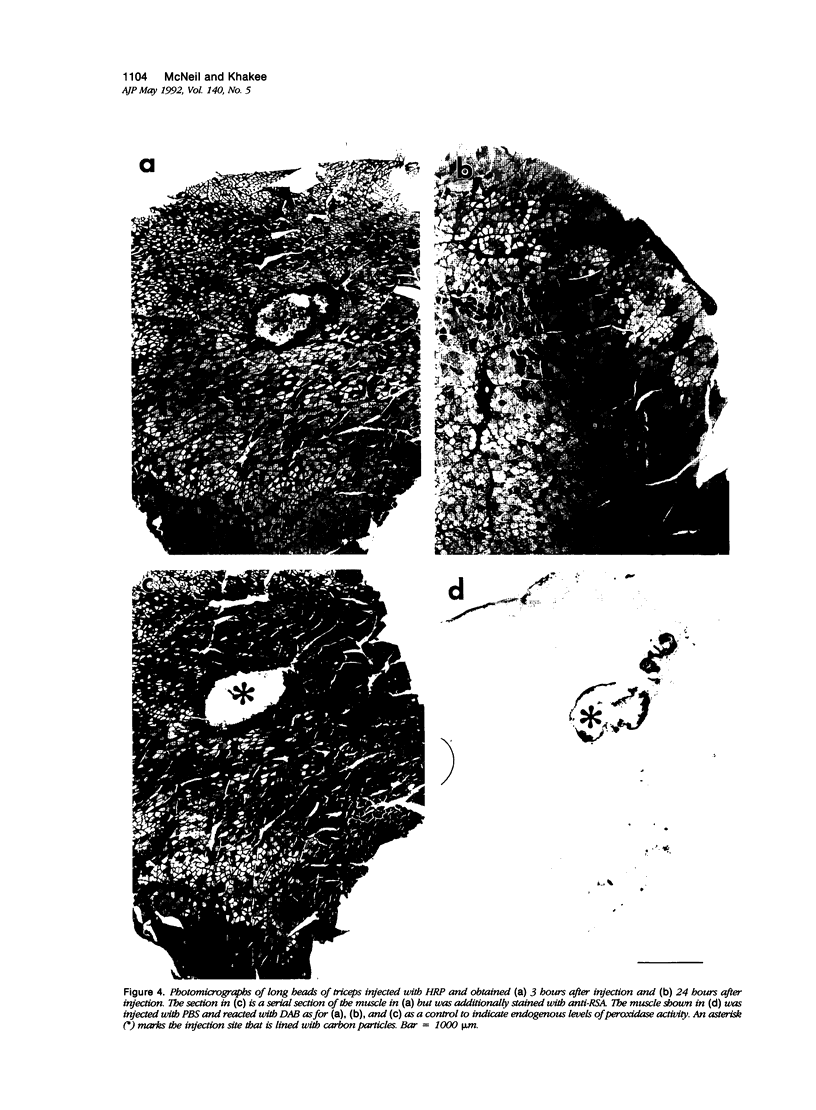
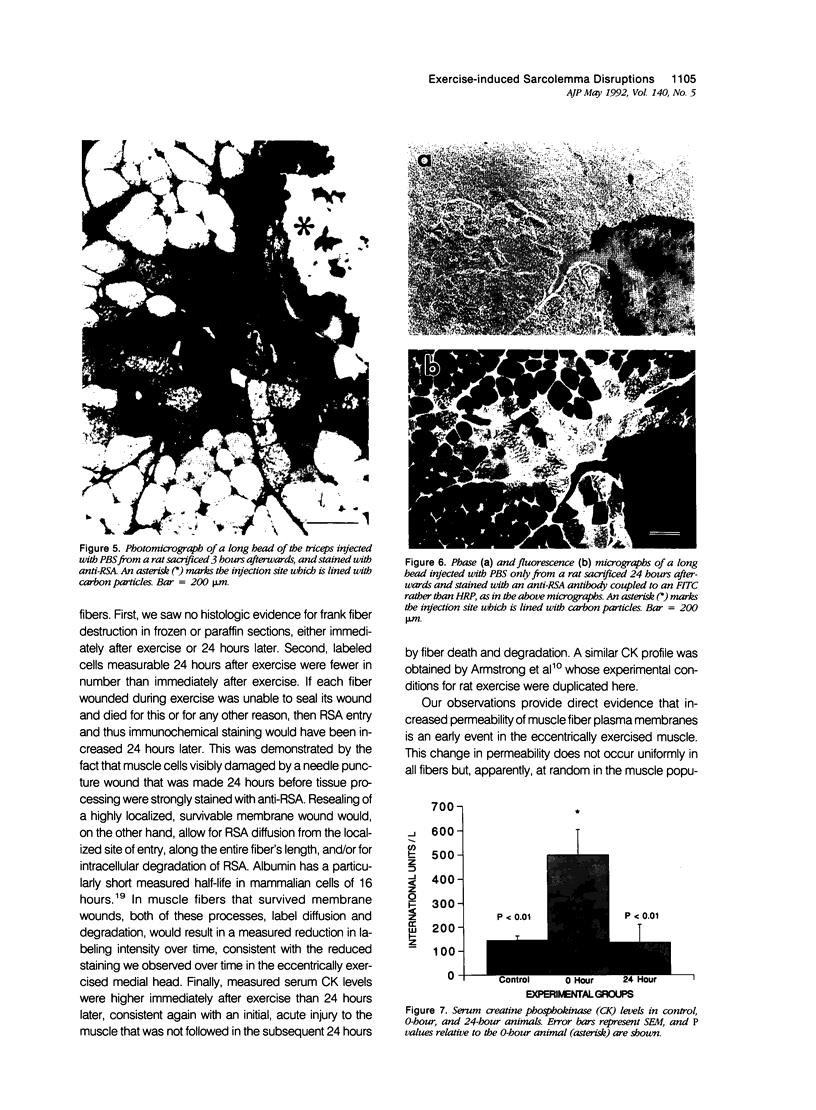
The authors have tested the hypothesis that plasma membrane disruptions are an early form of structural damage to the fibers of eccentrically exercised muscle. Rat serum albumin (RSA) was used as a marker for muscle-fiber wounding in the rat tricep (medial head) exercised eccentrically by downhill running. In all muscles examined, strong staining with a horseradish peroxidase (HRP)-conjugated anti-RSA antibody was observed between fibers (intercellular staining) and also within certain fibers (intracellular staining). This intracellular staining was interpreted as identifying muscle fibers wounded at their plasma membranes and hence rendered transiently or permanently permeable to extracellular RSA. The most striking finding of this study was a 6.9-fold increase relative to unexercised controls in the number of wounded cells in the medial head immediately after eccentric exercise. The authors also reproducibly observed, albeit less frequently, myocytes that stained with anti-RSA in the medial head and several other muscles of the "unexercised," caged laboratory rat. The extreme vulnerability of muscle plasma membranes to mechanically induced stress was revealed in this study by HRP injections into the triceps long head. A single injection of 200 microliters HRP through a 26-gauge needle resulted in extensive labeling of the muscle fibers present in the long head cross-sectioned at the injection site. The authors propose that initially resealable and/or highly localized, unsealable membrane wounds are an early form of exercise-induced damage that could progress along the length of the fiber until, 1 to 4 days after eccentric exercise, it becomes sufficiently severe that it can be readily recognized as the frank fiber necrosis and cellular infiltration described in numerous previous studies. In possessing cells wounded at their plasma membranes, normal, undisturbed rat muscle and eccentrically exercised muscle appears to resemble gut and skin, two additional tissues routinely exposed to mechanical forces in vivo. The authors propose that membrane disruptions provide a route into and out of myofiber cytoplasm distinct from the conventional, membrane-bounded routes of endo- and exocytosis, and therefore may be of importance both technically, as a route for introducing foreign genes into muscle cells, and biologically, as a route for release of the growth factor, basic fibroblast growth factor.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. C. Heavy metal intensification of DAB-based HRP reaction product. J Histochem Cytochem. 1981 Jun;29(6):775–775. doi: 10.1177/29.6.7252134. [DOI] [PubMed] [Google Scholar]
- Armstrong R. B., Ogilvie R. W., Schwane J. A. Eccentric exercise-induced injury to rat skeletal muscle. J Appl Physiol Respir Environ Exerc Physiol. 1983 Jan;54(1):80–93. doi: 10.1152/jappl.1983.54.1.80. [DOI] [PubMed] [Google Scholar]
- Byrnes W. C., Clarkson P. M., White J. S., Hsieh S. S., Frykman P. N., Maughan R. J. Delayed onset muscle soreness following repeated bouts of downhill running. J Appl Physiol (1985) 1985 Sep;59(3):710–715. doi: 10.1152/jappl.1985.59.3.710. [DOI] [PubMed] [Google Scholar]
- DiMario J., Buffinger N., Yamada S., Strohman R. C. Fibroblast growth factor in the extracellular matrix of dystrophic (mdx) mouse muscle. Science. 1989 May 12;244(4905):688–690. doi: 10.1126/science.2717945. [DOI] [PubMed] [Google Scholar]
- Fechheimer M., Boylan J. F., Parker S., Sisken J. E., Patel G. L., Zimmer S. G. Transfection of mammalian cells with plasmid DNA by scrape loading and sonication loading. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8463–8467. doi: 10.1073/pnas.84.23.8463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridén J., Sjöström M., Ekblom B. Myofibrillar damage following intense eccentric exercise in man. Int J Sports Med. 1983 Aug;4(3):170–176. doi: 10.1055/s-2008-1026030. [DOI] [PubMed] [Google Scholar]
- Gimlich R. L., Braun J. Improved fluorescent compounds for tracing cell lineage. Dev Biol. 1985 Jun;109(2):509–514. doi: 10.1016/0012-1606(85)90476-2. [DOI] [PubMed] [Google Scholar]
- Johnson G. D., Nogueira Araujo G. M. A simple method of reducing the fading of immunofluorescence during microscopy. J Immunol Methods. 1981;43(3):349–350. doi: 10.1016/0022-1759(81)90183-6. [DOI] [PubMed] [Google Scholar]
- Johnson S. E., Allen R. E. The effects of bFGF, IGF-I, and TGF-beta on RMo skeletal muscle cell proliferation and differentiation. Exp Cell Res. 1990 Apr;187(2):250–254. doi: 10.1016/0014-4827(90)90088-r. [DOI] [PubMed] [Google Scholar]
- Jones D. A., Newham D. J., Round J. M., Tolfree S. E. Experimental human muscle damage: morphological changes in relation to other indices of damage. J Physiol. 1986 Jun;375:435–448. doi: 10.1113/jphysiol.1986.sp016126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuipers H., Drukker J., Frederik P. M., Geurten P., van Kranenburg G. Muscle degeneration after exercise in rats. Int J Sports Med. 1983 Feb;4(1):45–51. doi: 10.1055/s-2008-1026015. [DOI] [PubMed] [Google Scholar]
- Lieber R. L., Fridén J. Selective damage of fast glycolytic muscle fibres with eccentric contraction of the rabbit tibialis anterior. Acta Physiol Scand. 1988 Aug;133(4):587–588. doi: 10.1111/j.1748-1716.1988.tb08446.x. [DOI] [PubMed] [Google Scholar]
- Lijnen P., Hespel P., Fagard R., Lysens R., Vanden Eynde E., Goris M., Goossens W., Lissens W., Amery A. Indicators of cell breakdown in plasma of men during and after a marathon race. Int J Sports Med. 1988 Apr;9(2):108–113. doi: 10.1055/s-2007-1024989. [DOI] [PubMed] [Google Scholar]
- McNeil P. L., Ito S. Gastrointestinal cell plasma membrane wounding and resealing in vivo. Gastroenterology. 1989 May;96(5 Pt 1):1238–1248. doi: 10.1016/s0016-5085(89)80010-1. [DOI] [PubMed] [Google Scholar]
- McNeil P. L., Ito S. Molecular traffic through plasma membrane disruptions of cells in vivo. J Cell Sci. 1990 Jul;96(Pt 3):549–556. doi: 10.1242/jcs.96.3.549. [DOI] [PubMed] [Google Scholar]
- McNeil P. L., Murphy R. F., Lanni F., Taylor D. L. A method for incorporating macromolecules into adherent cells. J Cell Biol. 1984 Apr;98(4):1556–1564. doi: 10.1083/jcb.98.4.1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil P. L., Muthukrishnan L., Warder E., D'Amore P. A. Growth factors are released by mechanically wounded endothelial cells. J Cell Biol. 1989 Aug;109(2):811–822. doi: 10.1083/jcb.109.2.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow N. G., Kraus W. E., Moore J. W., Williams R. S., Swain J. L. Increased expression of fibroblast growth factors in a rabbit skeletal muscle model of exercise conditioning. J Clin Invest. 1990 Jun;85(6):1816–1820. doi: 10.1172/JCI114640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukrishnan L., Warder E., McNeil P. L. Basic fibroblast growth factor is efficiently released from a cytolsolic storage site through plasma membrane disruptions of endothelial cells. J Cell Physiol. 1991 Jul;148(1):1–16. doi: 10.1002/jcp.1041480102. [DOI] [PubMed] [Google Scholar]
- Newham D. J., Jones D. A., Clarkson P. M. Repeated high-force eccentric exercise: effects on muscle pain and damage. J Appl Physiol (1985) 1987 Oct;63(4):1381–1386. doi: 10.1152/jappl.1987.63.4.1381. [DOI] [PubMed] [Google Scholar]
- Newham D. J., Jones D. A., Edwards R. H. Large delayed plasma creatine kinase changes after stepping exercise. Muscle Nerve. 1983 Jun;6(5):380–385. doi: 10.1002/mus.880060507. [DOI] [PubMed] [Google Scholar]
- Newham D. J., Jones D. A., Edwards R. H. Plasma creatine kinase changes after eccentric and concentric contractions. Muscle Nerve. 1986 Jan;9(1):59–63. doi: 10.1002/mus.880090109. [DOI] [PubMed] [Google Scholar]
- Newham D. J., McPhail G., Mills K. R., Edwards R. H. Ultrastructural changes after concentric and eccentric contractions of human muscle. J Neurol Sci. 1983 Sep;61(1):109–122. doi: 10.1016/0022-510x(83)90058-8. [DOI] [PubMed] [Google Scholar]
- Ogilvie R. W., Armstrong R. B., Baird K. E., Bottoms C. L. Lesions in the rat soleus muscle following eccentrically biased exercise. Am J Anat. 1988 Aug;182(4):335–346. doi: 10.1002/aja.1001820405. [DOI] [PubMed] [Google Scholar]
- Rote K. V., Rechsteiner M. Degradation of microinjected proteins: effects of lysosomotropic agents and inhibitors of autophagy. J Cell Physiol. 1983 Jul;116(1):103–110. doi: 10.1002/jcp.1041160116. [DOI] [PubMed] [Google Scholar]
- Round J. M., Jones D. A., Cambridge G. Cellular infiltrates in human skeletal muscle: exercise induced damage as a model for inflammatory muscle disease? J Neurol Sci. 1987 Dec;82(1-3):1–11. doi: 10.1016/0022-510x(87)90002-5. [DOI] [PubMed] [Google Scholar]
- Stauber W. T. Eccentric action of muscles: physiology, injury, and adaptation. Exerc Sport Sci Rev. 1989;17:157–185. [PubMed] [Google Scholar]
- Tidball J. G., Chan M. Adhesive strength of single muscle cells to basement membrane at myotendinous junctions. J Appl Physiol (1985) 1989 Sep;67(3):1063–1069. doi: 10.1152/jappl.1989.67.3.1063. [DOI] [PubMed] [Google Scholar]
- Tidball J. G., Law D. J. Dystrophin is required for normal thin filament-membrane associations at myotendinous junctions. Am J Pathol. 1991 Jan;138(1):17–21. [PMC free article] [PubMed] [Google Scholar]
- Trotter J. A., Eberhard S., Samora A. Structural domains of the muscle-tendon junction. 1. The internal lamina and the connecting domain. Anat Rec. 1983 Dec;207(4):573–591. doi: 10.1002/ar.1092070406. [DOI] [PubMed] [Google Scholar]
- Wolff J. A., Malone R. W., Williams P., Chong W., Acsadi G., Jani A., Felgner P. L. Direct gene transfer into mouse muscle in vivo. Science. 1990 Mar 23;247(4949 Pt 1):1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- Yamada S., Buffinger N., DiMario J., Strohman R. C. Fibroblast growth factor is stored in fiber extracellular matrix and plays a role in regulating muscle hypertrophy. Med Sci Sports Exerc. 1989 Oct;21(5 Suppl):S173–S180. [PubMed] [Google Scholar]